Role of microRNA-34 cluster and its therapeutic potential: a review of the literature
-
摘要:
MicroRNA-34(miR-34)家族包括miR-34a、miR-34b、miR-34c,与人体正常发育及多个器官功能的维持密切相关。MiR-34的表达受抑癌基因p53和长链非编码RNA(long non-coding RNAs,lncRNAs)的调控,其表达失衡会导致一系列疾病的发生发展。MiR-34与肿瘤发生发展密切相关,主要通过阻碍细胞周期、诱导细胞凋亡与自噬、抑制上皮-间充质转化、阻止细胞迁移与侵袭等机制,发挥抑癌作用。MiR-34在心脏保护中也发挥着重要作用。MiR-34功能的发挥主要涉及NF-κB、Notch等多个信号通路,基于miR-34表达的调控有利于部分疾病的控制和转归,是潜在的临床诊治靶标,具有广阔的临床应用前景。因此,本文就miR-34家族的功能及应用进展进行综述,以期为后续研究提供参考。
Abstract:MicroRNA-34 (miR-34) cluster includes miR-34a, miR-34b and miR-34c, which are closely related to the normal development of human body and the maintenance of multiple organ functions. The expression of miR-34 is regulated by tumor suppressor gene p53 and long non-coding RNAs (lncRNAs). Its unbalanced expression will lead to the occurrence and development of a series of diseases. MiR-34 is closely related to tumorigenesis and development. It plays an anti-cancer role mainly by blocking cell cycle, inducing apoptosis and autophagy, inhibiting epithelial mesenchymal transformation, and preventing cell migration and invasion. There is new evidence that miR-34 also plays an important role in cardiac protection. The function of miR-34 mainly involves NF-κB, Notch and other signaling pathways. The regulation based on the expression of miR-34 is conducive to the control and prognosis of some diseases. miR-34 is a potential target for clinical diagnosis and treatment, and has broad clinical application prospects. Therefore, this paper reviews the function and application progress of miR-34 cluster to provide reference for follow-up research.
-
Keywords:
- miR-34 cluster /
- p53 /
- lncRNA /
- tumor inhibition /
- cardiac protection /
- signaling pathways
-
MicroRNA(miRNA)是一种单链非编码RNA,长约22个核苷酸。MiRNA广泛存在于真核生物中,参与基因的转录后调控表达,其机理为通过与3'端非翻译区(3'-UTR)结合,导致目标mRNA降解或翻译抑制。MicroRNA-34(miR-34)家族由3个进化保守的miRNAs组成:miR-34a、miR-34b和miR-34c[1]。
MiR-34家族与抑癌基因p53间存在密切联系,miR-34不仅受p53去甲基化等方式调节,还通过其他调控因子形成正反馈环。迄今为止,许多研究[2-7]集中于miR-34家族在恶性肿瘤中的作用,其在多种癌症中均呈现低表达,起抑癌作用,主要通过阻碍细胞周期、促进细胞凋亡、抑制上皮-间充质转化(epithelial-mesenchymal transition,EMT)、阻止细胞迁移与侵袭的方式发挥功能。进一步研究后发现,miR-34家族在心脏保护中也发挥一定功能,其诱导心肌成纤维细胞增殖,并在心肌重构和梗死后修复中发挥重要作用,还参与多个信号通路(NF-κB、Notch等)的调节。本文就miR-34家族的功能及临床应用进展进行综述,以期为进一步深入研究提供参考。
1. MiR-34家族组成及生物学特征
MiR-34家族是一类进化中高度保守的miRNA家族,广泛存在于节肢动物、线虫纲动物及脊椎动物中。2003年,Houbaviy等[8]从小鼠胚胎干细胞中克隆了3个紧密相关的基因,并将其命名为miR-34a、miR-34b和miR-172。这些miRNAs后被更名为miR-34c、miR-34b和miR-34a,以阐明与人序列的同源性。
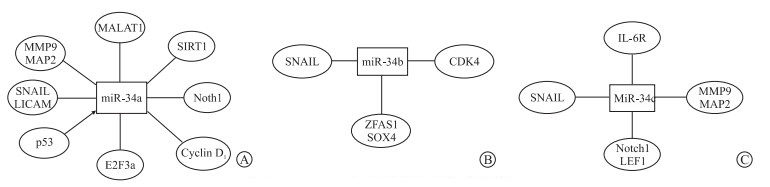
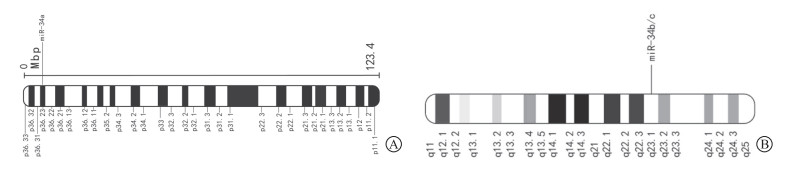
人体中有2个基因编码miR-34家族,其中miR-34a的基因座位于染色体1p36上(图 1A),而miR-34b和miR-34c则由染色体11q23上编码的多顺反子转录物表达(图 1B)。哺乳动物中,miR-34的3个家族成员在不同组织中的表达具有特异性,在各细胞系和组织中,miR-34的丰度直接受p53调控[9]。
2. MiR-34家族的生物学功能及调控机制
2.1 p53途径
MiR-34家族在抑制肿瘤中发挥重要作用。2007年,He等[10]首次发现,miR-34家族与重要的抑癌基因p53具有密切关系。2010年,miR-34a/b/c被认为是直接的p53靶点,通过下调干细胞相关基因显著抑制体细胞重编程[2]。
MiR-34家族中miR-34a常见于p53的调节,当细胞受到诸如DNA损伤的刺激时,依赖于p53的miR-34a表达被激活,随后诱导细胞周期阻滞、促进凋亡或DNA修复[11]。
MiR-34a的启动子包含1个p53结合位点和1个CpG岛,两者的甲基化导致miR-34a下调。启动子缺失或突变以及p53失活是miR-34a的沉默机制之一[12-13]。p53可使miR-34的CpG岛启动子区域去甲基化从而激活miR-34表达。研究[14]表明,p53蛋白直接靶向调节miR-34a的表达。此外,也有间接调控因子如沉默信息调节因子相关酶1(SIRT1)、MAGE-A和HDM4影响p53与miR-34的表达。SIRT1是一种依赖NAD的去乙酰化酶,miR-34a降低SIRT1水平,促进p53乙酰化和活化,进而促miR-34a表达[15]。MiR-34a还可抑制MAGE-A基因的某些成员,进而促进p53及其靶基因p21WAF1/CIP1的表达[16]。此外,miR-34a可直接抑制HDM4(p53的负调节因子),形成作用于p53的正反馈环[17]。MiR-34a抑制剂可减弱p53介导的细胞凋亡,以应对基因毒性应激,而miR-34a的异位表达可导致显著的基因表达重编程,并诱导细胞凋亡和细胞周期停滞[18]。
研究[1]表明,突变型p53样本中miR-34a/b/c的表达显著降低。p53/miR-34轴抑制SNAIL,而SNAIL诱导EMT。通过miR-34家族对SNAIL的抑制,可促进Met的产生,并消除癌细胞的恶性特征。miR-34c可通过p53-p21Cip1-细胞周期蛋白依赖性激酶(CDK)/cyclin或p53非依赖性CDK/cyclin途径诱导白血病干细胞衰老[19]。此外,有学者[20]发现p53诱导的miR-34a/b/c基因在结直肠癌中常被沉默,证明miR-34a/b/c缺陷型腺瘤增殖增强,凋亡减少。
2.2 长链非编码RNA调控途径
miR-34家族除了由p53调控表达外,也可以独立的方式被调控。MiR-34家族已被证明可通过TP63、TP73和其他转录因子(transcription factors,TFs)如STAT3、MYC和EMT TFs进行转录调控,并通过多种长链非编码RNA(lncRNAs)进行转录后调控[21]。
一些lncRNAs在表观遗传学上调节miR-34a的表达,例如lncRNA HOTAIR驱动成纤维细胞的EZH2甲基化,而EZH2可通过miRNA-34a甲基化增加Notch1转录[22]。此外,一些lncRNAs作为海绵或ceRNA调控miR-34家族。SNHG7作为miR-34a的海绵,可通过吸附miR-34a调控SYVN1(Synoviolin 1),最终影响细胞的增殖、凋亡和自噬。而miR-34a的过表达可逆转lncRNA SNHG7抑制细胞凋亡和自噬的效果[23]。LINC00346作为miR-34a的海绵发挥作用,通过保护Notch 1、AXL Receptor Tyrosine Kinase和CD44免受miR-34a介导的降解,从而促进胃癌中的癌细胞增殖、侵袭和转移[24]。在卵巢癌中,Lnc-OC1与miR-34a/c形成相互抑制的反馈环。OC1的下调可抑制细胞的增殖、侵袭、迁移和异种移植生长[25]。
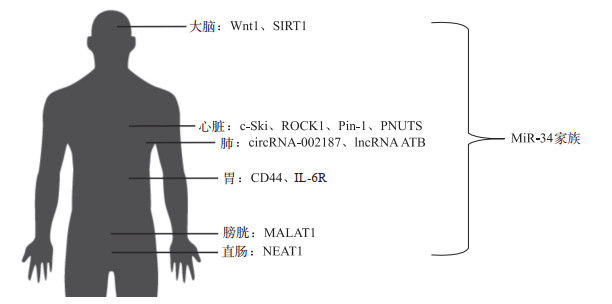
除癌症领域外,lncRNA-SNHG7作为ceRNA靶向miR-34以促进心肌纤维化。SNHG7的低表达将改善小鼠心肌梗死后的心功能。同时,miR-34可反向抑制SNHG7诱导的心脏纤维化及成纤维细胞向肌成纤维细胞的转化[26]。研究[22]发现,lncRNA-HOTAIR的过表达会抑制miRNA-34a的表达与Notch通路的激活,并且还可在体外增加胶原和α-SMA表达。
3. MiR-34家族调控失衡与疾病发生发展
3.1 MiR-34与肿瘤
2007年,Tarasov等[27]发现,在所有miRNAs中,miR-34家族对p53的诱导作用最为显著。后续研究[21]表明,p53在miR-34a启动子的邻近区域有多个结合位点,证实miR-34家族在多种癌症中呈低表达,起抑癌作用。
除p53突变外,miR-34a启动子的CpG甲基化[28],染色体臂1p缺失[29]也会导致miR-34a的下调,最终使miR-34的靶基因表达量增高。Deng等[30]证实抑癌基因miR-34b直接靶向ZFAS1,抑制ZFAS1的表达。癌基因SOX4也是miR-34b的直接下游靶点,沉默ZFAS1可以通过调节miR-34b来抑制SOX4。miR-34b可直接靶向ZFAS1并抑制大肠癌细胞的转移。miR-34c则可靶向Notch1和淋巴增强因子1(lymphoid enhancer-binding factor 1, LEF1)降低骨肉瘤转移和化疗耐药[31]。MiR-34家族对癌症的影响可从对抑制细胞周期、促进凋亡、抑制EMT过程、抑制迁移和侵袭的影响进行分析。
MiR-34家族可诱导细胞周期阻滞抑制肿瘤进程,如miR-34a抑制E2F3a的表达,而E2F3a是通过刺激和加速G1/S转换来激活细胞周期的关键因子[3]。Liu等[4]证明miR-34a受lncRNA MALAT1抑制,同时发现Cyclin D1为miR-34a的靶基因,miR-34a会抑制Cyclin D1在膀胱癌细胞中的表达,证明在膀胱癌中上调的MALAT1通过miR-34a/Cyclin D1轴可促进肿瘤细胞增殖和迁移。Feng等[32]发现miR-34b靶向CDK4,CDK4的过表达可解除miR-34的抑制效果,敲除miR-34b/c和miR-449可引起细胞周期相关基因如CDK1、Cyclin B1、Ccna1、Cdc20(Cell Division Cycle 20)上调,Cdc14a的表达水平下调,从而促进细胞的增殖[33]。
MiR-34过表达可诱导Notch1相关的凋亡和自噬,从而抑制卵巢癌细胞增殖[6]。过表达miR-34,凋亡相关蛋白(Bax)和自噬相关蛋白(LC3-Ⅱ和p62)的升高,通过激活凋亡和自噬导致卵巢癌细胞显著减少[6]。MiR-34还可通过SIRT1调控大肠癌细胞自噬与凋亡,GAS5/miR-34a/SIRT1/mTOR负调节反馈环介导大肠癌细胞的自噬使得miR-34在大肠癌的发展过程中起抗凋亡作用[34]。Qu等[35]同样通过SIRT1通路发现,miR-34a表达与CTRP6对TNF-α诱导的细胞凋亡的保护作用有关。近年研究[36]中,通过检测Bcl-2、Bax (Bcl-2 associated X protein)、Bak (Bcl-2 associated K protein)、caspase-9和survivin的相对表达,发现miR-34可通过TGF-β/Smads信号通路促进白内障大鼠晶状体上皮细胞凋亡。Anastasiadou等[37]发现,下调miR-34a会增加程序性死亡配体1(programmed death ligand 1, PD-L1)使得肿瘤T细胞的凋亡增加,致使B细胞淋巴瘤的致癌能力增强。
在EMT调节中,miR-34a下调SLUG和ZEB1及一些干细胞因子,如BMI1、CD44等。Chen等[5]证明CD44是miR-34a转录后水平抑制的靶点,miR-34a可能通过抑制CD44的表达而抑制神经母细胞瘤的进展。如前文所述,p53/miR-34轴抑制SNAIL,进而促进Met过程,并消除癌细胞的恶性特征[1]。Wu等[29]发现,miR-34a通过下调MET,从而抑制头颈鳞状细胞癌细胞增殖。MiR-34a还在子宫内膜癌中抑制膜糖蛋白L1CAM,而L1CAM与EMT密切相关[38]。
在宫颈癌中,miR-34a抑制细胞增殖、迁移和侵袭,同时基质金属蛋白酶9 (MMP9)活性和微管相关蛋白2(MAP2)含量下降[7]。而MAP2与上皮细胞中的迁移有关[39];MMP9在肿瘤细胞侵袭中起重要作用。MiR-34b抑制增殖和迁移,对MMP9和MAP2基因无影响。MiR-34c抑制增殖和迁移,且抑制MMP9活性和MAP2蛋白。研究[40]发现,miR-34c在胃癌细胞中抑制白细胞介素6受体(IL-6R)。人巨细胞病毒通过下调miR-34c调节IL-6/STAT3通路,可促进胃癌细胞的增殖和侵袭。Dehghan等[41]证实,miR-34a抑制TGF-β、CXCL-12和CCL-2,可减缓炎症,并使得细胞迁移和增殖能力下降。此外,NEAT1作为ceRNA抑制miR-34a/SIRT1轴和激活Wnt/β连环蛋白信号通路,从而影响直肠癌细胞增殖和转移[42]。
3.2 MiR-34与心脏保护
2012年,Iekushi等[43]发现,miR-34a是PD-1抑制剂治疗的心脏组织中最明显上调的miRNA,也是心脏组织中重要的不良反应物。2013年,Boon等[44]证实年龄诱导的miR-34a表达及其抑制PNUTS是通过诱导DNA损伤反应和端粒磨损来调节衰老期间和急性心肌梗死后心脏收缩功能的关键机制。PNUTS是miR-34a的直接靶点,可减少端粒缩短、DNA损伤反应和心肌细胞凋亡并改善急性心肌梗死后的功能恢复。使用miR-34a抑制剂可减少急性心肌梗死后的细胞死亡和纤维化,从而促进心肌功能的恢复[44]。Xia等[45-46]发现,miR-34a/PNUTS信号通路可在PD-1抑制剂处理的巨噬细胞产生的外泌体中发挥促衰老作用。MiR-34a还在PD-1抑制剂诱导的心肌损伤和巨噬细胞极化中起调节作用,通过靶向KLF4发挥抗炎效应及心脏保护作用。
MiR-34家族可通过TGF-β通路影响心脏纤维化进展,而TGF-β信号通路是心肌纤维化的关键调控因子。Huang等[47]的研究中,miR-34a通过TGF-β信号通路显著诱导心肌成纤维细胞增殖,且miR-34a水平与心脏成纤维细胞中TGF-β1的促纤维活性正相关。c-Ski是TGF-β信号转导的抑制性调节因子,过表达c-Ski可抑制细胞外基质蛋白的表达,并显著抑制人心肌成纤维细胞增殖。实验[48]证明,miR-34a-c-Ski轴可在体内外调节TGF-β1和异丙肾上腺素诱导的心肌纤维化。除c-Ski外,ROCK1也是TGF-β信号通路中的一个关键效应因子,介导分子开关RhoA的下游调节[49]。ROCK1的上调可促进成纤维细胞-肌成纤维细胞的转化以及胶原1和α-SMA的蛋白水平。Wang等[26]证明,miR-34具有心脏保护作用,并通过靶向ROCK1途径减轻心肌纤维化。lncRNA-SNHG7作为ceRNA通过靶向miR-34促进心肌纤维化,而miR-34可通过靶向ROCK1抑制心肌成纤维细胞的纤维化,并抑制SNHG7诱导的心肌成纤维细胞增殖和成纤维细胞-肌成纤维细胞转化,从而消除心脏重构,改善心脏功能。
Zhang等[50]研究表明,过表达miR-34b/c从而抑制心肌细胞的免疫功能,促进心肌细胞中促炎症细胞因子的分泌。MiR-34b/c可抑制ITCH并通过ITCH/NF-κB途径可保护心肌细胞免受阿霉素损伤。Giricz等[51]证明,大鼠中的miR-32与miR-34c与钙传感器Swiprosin-1的调控有关,而Swiprosin-1通过阻断cofilin的结合位点来稳定纤维状肌动蛋白,在心肌重构和梗死后修复中发挥作用。Pin-1参与了扩张型心肌病心肌重塑(心肌肥大、凋亡和纤维化)的过程,是心力衰竭发生的重要病理生理基础[52]。Zhang等[53]发现,miR-34a可靶向Pin-1信号并减少Ⅰ型胶原的产生、细胞活力和心肌梗死增加心脏成纤维细胞的凋亡来减轻扩张型心肌病的心肌纤维化。此外,miR-34a/b/c通过低SIRT1水平与冠心病的存在有显著的间接关联[54]。
4. MiR-34家族的作用途径及通路
4.1 NF-κB信号通路
在众多信号通路中,NF-κB信号通路与肿瘤的发生发展、免疫发育应答、炎症的激活与抑制密切相关。研究[55-58]显示miR-34家族与NF-κB信号通路具有密切关系。
近期研究[55]表明,miR-34a和miR-34c通过靶向LGR4增强表皮角质形成细胞的炎症反应,可通过增加p65磷酸化下调NF-kB信号活性。炎症调节因子如TNF-α、IL-6、IL-8及VEGF显著提高与NF-κB非经典激活途径密切相关[56]。而如前文所述,miR-34a显著降低这些炎症因子的表达[41]。表皮生长因子受体(epidermal growth factor receptor,EGFR)是miR-34a的直接靶标[59],且是NF-κB的促进因子。
研究[57]发现,miR-34受褪黑素抑制,并与抑制细胞凋亡基因Bcl-2的上调有关。褪黑素通过调节细胞因子的产生来控制先天性免疫反应,并通过降低NF-κB、p50和p65的表达抑制TNF-α和IL-6的表达[58]。SIRT1受miR-34a的抑制从而上调IL-1β/COX2/PGE2途径[60]。过表达miR-34a可增强IL-1β的表达,从而增强炎症;下调miR-34a则诱导SIRT1的表达,降低NF-κB下游转录因子的激活。抑制miR-34a则下调IL-1β、NF-κB p65和乙酰化NF-κB p65在mRNA和蛋白质水平的表达,表明其对炎症和NF-κB活化的抑制作用[60]。此外,如前文所述,在宫颈癌中发现miR-34a和miR-34c降低MMP9活性[7]。
4.2 Notch信号通路
Notch信号通路介导细胞间的信号传导来调节各种组织中细胞的命运决定,在多种类型的癌症中被激活[61]。Notch1的激活可促进多种肿瘤细胞的增殖,而Notch1沉默可诱导凋亡和自噬从而抑制卵巢癌细胞生长[6]。此处选取Notch通路中的关键分子包括Notch配体与受体、Bcl-2及相关蛋白Bax和Bak、survivin、Hes-1及RRAS(RAS Related)等进行讨论。
近年来,不断有研究探究miR-34家族在Notch信号通路中的具体调节机制。Zhang等[36]发现,miR-34模拟物转染大鼠晶状体上皮细胞后,Bax与Bak显著升高,而Bcl-2和survivin明显下降,细胞凋亡率升高。Liu等[62]测得miR-34a的过表达会抑制转染细胞中Notch受体Notch1、Notch2、Notch3和下游蛋白Hes-1的表达,从而影响子痫前期的发生。Chevalier等[63]发现,miR-34/449家族在多囊细胞分化过程中抑制Notch通路。在研究中,使用保护性寡核苷酸阻止miR-34/449与Notch1或Notch配体Dll1的结合,会协调性阻断多细胞发生和顶端肌动蛋白网络的形成。过表达miR-34a还会导致活性RhoA GTP水平显著提升。miR-34/449和R-Ras参与肌动蛋白网络重组,并具有改变RhoA活性的能力,而RhoA同样是Notch通路中的关键分子之一。Zhang等[64]发现,Notch1和Notch2的表达水平与miR-34b的表达水平负相关,miR-34b对视网膜母细胞瘤细胞起抑制效果。当miR-34b与Notch1或Notch2共转染后,这些表型可通过逆转细胞生长和肿瘤球体进程而被回调。
4.3 其他信号通路
除NF-κB、Notch信号通路外,miR-34家族还涉及SIRT[34-35, 42]、STAT[40]、Wnt[42]等其他信号通路,说明其广泛参与体内调控,并发挥重要作用。
5. 基于miR-34家族靶标的临床应用
5.1 降低耐药性
鉴于核酸为基础的药物具有潜在的免疫原性、体内不稳定性、脱靶效应和高细胞毒性等不良作用[65],miR-34a模拟物(miR-34 mimic)由于稳定、易产生、可同时抑制多种致癌途径,通常被认为是癌症治疗的更好选择[66]。
肿瘤干细胞(cancer stem cells, CSCs)数量的增加与阻碍化疗成功的化疗耐药有直接的关系,并被认为与治疗耐药性、复发和转移相关[67]。研究[68]发现,miR-34a表达减少的细胞中Notch1水平显著增加。重组miR-34a后干细胞减少,化疗敏感性提高。Lin等[66]发现,上调肿瘤细胞中miR-34a的表达,对紫杉烷耐药的前列腺癌细胞增殖抑制率为60%。在软骨肉瘤细胞中,miR-34通过活性氧(ROS)的积累以及CSCs和非CSCs之间动态平衡的干扰,导致肿瘤干细胞的放射敏感性增加[69]。
5.2 作为诊断标记物
由于miRNAs具有在肿瘤组织和血清中的高组织特异性与组织中高稳定性,miRNAs可作为极富前景的诊断标志物。Zhao等[13]发现非小细胞肺癌患者血浆中miR-34a水平越高,淋巴结转移的发生率越低,且血浆中miR-34a/c的高表达水平与更好的无病生存率和患者总体生存率间有很强的一致性。Hanafi等[70]也在研究中发现,miR-34在非小细胞肺癌患者的血清中高表达,可作为晚期非小细胞肺癌M1b转移、腺癌细胞类型及腺癌阴性EGFR突变的预测指标。
Agostini等[71]认为miR-34表达的降低与不良预后和生存率相关。几个miR-34a/b验证靶点的表达,包括Bcl-2、CCND1、E2F3、Met、CD44,与不同乳腺癌数据集的生存率相关。相反,低水平的miR-34a是人类肝细胞癌的积极预后因素。
除肿瘤外,miR-34家族还在其他领域中具有诊断标志物潜力。例如,miR-34/449表达模式的改变可导致输出小管上皮纤毛发生缺陷,导致进行性生精失败。Chevalier等[63]认为,miR-34/449不仅可用于区分可生育男性和不育男性,而且可用于无创准确诊断男性不育病因。Sessa等[72]研究表明,miR-34可用作吸毒(可卡因)致脑损伤的标记物。最新的研究[54]成果中还发现,miR-34a/b/c通过低SIRT1水平与冠心病的存在有显著的间接关联,miR-34家族可能是潜在的冠心病标记物。
综上所述,MiR-34家族包括miR-34a,miR-34b,miR-34c,该家族主要受p53去甲基化激活或通过其他调节因子形成正反馈环调控,其还受其他非编码RNAs(主要是lncRNAs)的调控,从而在体内发挥重要作用。研究[28-30]表明,miR-34家族的调控失衡与疾病发生发展具有密切联系。miR-34家族在癌症中主要发挥抑癌作用,包括阻碍细胞周期、诱导细胞凋亡与自噬、抑制上皮-间充质转化、阻止细胞迁移与侵袭。此外,miR-34家族还参与心脏保护的调节,可诱导心肌成纤维细胞增殖,并在心肌重构和梗死后修复中发挥作用,其具体调节功能通过多个信号通路(NF-κB、Notch等)实现。本文介绍基于miR-34家族靶标的临床应用,主要着重于其降低耐药性与作为诊断标记物2个方面,综述miR-34家族目前的研究进展及在药物研发中的趋势和前景。
然而,miR-34家族表达与患者生存率之间的相关性并不足以支持肿瘤抑制作用,还需对miR-34进行进一步研究以更好得描述其调节方式及其下游途径。MiRNA的治疗仍存在传递系统与脱靶效应的挑战。如使用纳米载体运送miR-34a时,还需商讨纳米粒子的毒性。此外,血清蛋白在纳米载体表面的结合,用于治疗的miRNA在健康组织中积聚,而流动血液产生的剪切应力会使纳米颗粒破裂,最终纳米载体未能成功渗到靶细胞等[73]。在实际应用过程中,运送miR-34a的脂质体在正常人体pH值下为阴离子,但在肿瘤微环境中(pH值较低)变成了阳离子。因此,后续研究应通过该特征增强肿瘤特异性摄取的能力。
在未来临床医学上,miRNA-34家族在卫生与健康领域中具有巨大的应用潜力,通过对其的研究与归纳,可以为肿瘤与心脏的研究方向给予全新的研究途径。
利益冲突:所有作者声明不存在利益冲突。 -
[1] CORNEY D C, HWANG C I, MATOSO A, et al. Frequent downregulation of miR-34 family in human ovarian cancers[J]. Clin Cancer Res, 2010, 16(4): 1119-1128. DOI: 10.1158/1078-0432.CCR-09-2642
[2] CHRISTOFFERSEN N R, SHALGI R, FRANKEL L B, et al. p53-independent upregulation of miR-34a during oncogene-induced senescence represses MYC[J]. Cell Death Differ, 2010, 17(2): 236-245. DOI: 10.1038/cdd.2009.109
[3] REIMER D, HUBALEK M, KIEFEL H, et al. Regulation of transcription factor E2F3a and its clinical relevance in ovarian cancer[J]. Oncogene, 2011, 30(38): 4038-4049. DOI: 10.1038/onc.2011.119
[4] LIU Y, GAO S, DU Q, et al. Knockdown of long non-coding RNA metastasis associated lung adenocarcinoma transcript 1 inhibits the proliferation and migration of bladder cancer cells by modulating the microRNA-34a/cyclin D1 axis[J]. Int J Mol Med, 2019, 43(1): 547-556.
[5] CHEN J, HONGTING L, SHAOPING L, et al. MiR-34-a acts as a suppressor in neuroblastoma progression by targeting CD44[J]. J Pak Med Assoc, 2017, 67(10): 1524-1531.
[6] JIA Y, LIN R, JIN H, et al. MicroRNA-34 suppresses proliferation of human ovarian cancer cells by triggering autophagy and apoptosis and inhibits cell invasion by targeting Notch 1[J]. Biochimie, 2019, 160: 193-199. DOI: 10.1016/j.biochi.2019.03.011
[7] CÓRDOVA-RIVAS S, FRAIRE-SOTO I, MERCADO-CASAS TORRES A, et al. 5p and 3p strands of miR-34 family members have differential effects in cell proliferation, migration, and invasion in cervical cancer cells[J]. Int J Mol Sci, 2019, 20(3): 545. DOI: 10.3390/ijms20030545
[8] HOUBAVIY H B, MURRAY M F, SHARP P A. Embryonic stem cell-specific MicroRNAs[J]. Dev Cell, 2003, 5(2): 351-358. DOI: 10.1016/S1534-5807(03)00227-2
[9] BOMMER G T, GERIN I, FENG Y, et al. p53-mediated activation of miRNA34 candidate tumor-suppressor genes[J]. Curr Biol, 2007, 17(15): 1298-1307. DOI: 10.1016/j.cub.2007.06.068
[10] HE L, HE X, LIM L P, et al. A microRNA component of the p53 tumour suppressor network[J]. Nature, 2007, 447(7148): 1130-1134. DOI: 10.1038/nature05939
[11] STANKEVICINS L, ALMEIDA DA SILVA A P, VENTURA DOS PASSOS F, et al. MiR-34a is up-regulated in response to low dose, low energy X-ray induced DNA damage in breast cells[J]. Radiat Oncol, 2013, 8: 231. DOI: 10.1186/1748-717X-8-231
[12] MA W, XIAO G G, MAO J, et al. Dysregulation of the miR-34a-SIRT1 axis inhibits breast cancer stemness[J]. Oncotarget, 2015, 6(12): 10432-10444. DOI: 10.18632/oncotarget.3394
[13] ZHAO K, CHENG J, CHEN B, et al. Circulating microRNA-34 family low expression correlates with poor prognosis in patients with non-small cell lung cancer[J]. J Thorac Dis, 2017, 9(10): 3735-3746. DOI: 10.21037/jtd.2017.09.01
[14] HERMEKING H. p53 enters the microRNA world[J]. Cancer Cell, 2007, 12(5): 414-418. DOI: 10.1016/j.ccr.2007.10.028
[15] NAGHIZADEH S, MOHAMMADI A, DUIJF P H G, et al. The role of miR-34 in cancer drug resistance[J]. J Cell Physiol, 2020, 235(10): 6424-6440. DOI: 10.1002/jcp.29640
[16] WEERARATNE S D, AMANI V, NEISS A, et al. miR-34a confers chemosensitivity through modulation of MAGE-A and p53 in medulloblastoma[J]. Neuro Oncol, 2011, 13(2): 165-175. DOI: 10.1093/neuonc/noq179
[17] OKADA N, LIN C P, RIBEIRO M C, et al. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression[J]. Genes Dev, 2014, 28(5): 438-450. DOI: 10.1101/gad.233585.113
[18] JIANG D, LI M, YU Y, et al. microRNA-34a aggravates coxsackievirus B3-induced apoptosis of cardiomyocytes through the SIRT1-p53 pathway[J]. J Med Virol, 2019, 91(9): 1643-1651. DOI: 10.1002/jmv.25482
[19] PENG D, WANG H, LI L, et al. miR-34c-5p promotes eradication of acute myeloid leukemia stem cells by inducing senescence through selective RAB27B targeting to inhibit exosome shedding[J]. Leukemia, 2018, 32(5): 1180-1188. DOI: 10.1038/s41375-018-0015-2
[20] JIANG L, HERMEKING H. miR-34a and miR-34b/c suppress intestinal tumorigenesis[J]. Cancer Res, 2017, 77(10): 2746-2758. DOI: 10.1158/0008-5472.CAN-16-2183
[21] SLABÁKOVÁ E, CULIG Z, REMŠÍK J, et al. Alternative mechanisms of miR-34a regulation in cancer[J]. Cell Death Dis, 2017, 8(10): e3100. DOI: 10.1038/cddis.2017.495
[22] WASSON C W, ABIGNANO G, HERMES H, et al. Long non-coding RNA HOTAIR drives EZH2-dependent myofibroblast activation in systemic sclerosis through miRNA 34a-dependent activation of NOTCH[J]. Ann Rheum Dis, 2020, 79(4): 507-517. DOI: 10.1136/annrheumdis-2019-216542
[23] TIAN F, WANG J, ZHANG Z, et al. LncRNA SNHG7/miR-34a-5p/SYVN1 axis plays a vital role in proliferation, apoptosis and autophagy in osteoarthritis[J]. Biol Res, 2020, 53(1): 9. DOI: 10.1186/s40659-020-00275-6
[24] XU T P, MA P, WANG W Y, et al. KLF5 and MYC modulated LINC00346 contributes to gastric cancer progression through acting as a competing endogeous RNA and indicates poor outcome[J]. Cell Death Differ, 2019, 26(11): 2179-2193. DOI: 10.1038/s41418-018-0236-y
[25] TAO F, TIAN X, LU M, et al. A novel lncRNA, Lnc-OC1, promotes ovarian cancer cell proliferation and migration by sponging miR-34a and miR-34c[J]. J Genet Genomics, 2018, 45(3): 137-145. DOI: 10.1016/j.jgg.2018.03.001
[26] WANG J, ZHANG S, LI X, et al. LncRNA SNHG7 promotes cardiac remodeling by upregulating ROCK1 via sponging miR-34-5p[J]. Aging (Albany NY), 2020, 12(11): 10441-10456.
[27] TARASOV V, JUNG P, VERDOODT B, et al. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest[J]. Cell Cycle, 2007, 6(13): 1586-1593. DOI: 10.4161/cc.6.13.4436
[28] SIEMENS H, NEUMANN J, JACKSTADT R, et al. Detection of miR-34a promoter methylation in combination with elevated expression of c-Met and beta-catenin predicts distant metastasis of colon cancer[J]. Clin Cancer Res, 2013, 19(3): 710-720.
[29] WU X, CHENG Y L, MATTHEN M, et al. Down-regulation of the tumor suppressor miR-34a contributes to head and neck cancer by up-regulating the MET oncogene and modulating tumor immune evasion[J]. J Exp Clin Cancer Res, 2021, 40(1): 70. DOI: 10.1186/s13046-021-01865-2
[30] DENG H, WANG M, XU Q, et al. ZFAS1 promotes colorectal cancer metastasis through modulating miR-34b/SOX4 targeting[J]. Cell Biochem Biophys, 2021, 79(2): 387-396. DOI: 10.1007/s12013-021-00976-z
[31] XU M, JIN H, XU C X, et al. MiR-34c inhibits osteosarcoma metastasis and chemoresistance[J]. Med Oncol, 2014, 31(6): 972. DOI: 10.1007/s12032-014-0972-x
[32] FENG H, GE F, DU L, et al. MiR-34b-3p represses cell proliferation, cell cycle progression and cell apoptosis in non-small-cell lung cancer (NSCLC) by targeting CDK4[J]. J Cell Mol Med, 2019, 23(8): 5282-5291. DOI: 10.1111/jcmm.14404
[33] WU Y J, LIU Y, HU Y Q, et al. Control of multiciliogenesis by miR-34/449 in the male reproductive tract through enforcing cell cycle exit[J]. J Cell Sci, 2021, 134(9): jcs253450. DOI: 10.1242/jcs.253450
[34] ZHANG H G, WANG F J, WANG Y, et al. lncRNA GAS5 inhibits malignant progression by regulating macroautophagy and forms a negative feedback regulatory loop with the miR34a/mTOR/SIRT1 pathway in colorectal cancer[J]. Oncol Rep, 2021, 45(1): 202-216.
[35] QU L H, HONG X, ZHANG Y, et al. C1q/tumor necrosis factor-related protein-6 attenuates TNF-alpha-induced apoptosis in salivary acinar cells via AMPK/SIRT1-modulated miR-34a-5p expression[J]. J Cell Physiol, 2021, 236(8): 5785-5800. DOI: 10.1002/jcp.30262
[36] ZHANG G B, LIU Z G, WANG J, et al. MiR-34 promotes apoptosis of lens epithelial cells in cataract rats via the TGF-beta/Smads signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2020, 24(7): 3485-3491.
[37] ANASTASIADOU E, STROOPINSKY D, ALIMPERTI S, et al. Epstein-Barr virus-encoded EBNA2 alters immune checkpoint PD-L1 expression by downregulating miR-34a in B-cell lymphomas[J]. Leukemia, 2019, 33(1): 132-147. DOI: 10.1038/s41375-018-0178-x
[38] SCHIRMER U, DOBERSTEIN K, RUPP A K, et al. Role of miR-34a as a suppressor of L1CAM in endometrial carcinoma[J]. Oncotarget, 2014, 5(2): 462-472. DOI: 10.18632/oncotarget.1552
[39] LIU S Y, CHEN Y T, TSENG M Y, et al. Involvement of microtubule-associated protein 2(MAP2) in oral cancer cell motility: a novel biological function of MAP2 in non-neuronal cells[J]. Biochem Biophys Res Commun, 2008, 366(2): 520-525. DOI: 10.1016/j.bbrc.2007.11.179
[40] SHI L, FAN B, CHEN D, et al. Human cytomegalovirus protein UL136 activates the IL-6/STAT3 signal through MiR-138 and MiR-34c in gastric cancer cells[J]. Int J Clin Oncol, 2020, 25(11): 1936-1944. DOI: 10.1007/s10147-020-01749-z
[41] DEHGHAN R, NAJAFI R, AZIZI JALILIAN F, et al. A promising effect of zerumbone with improved anti-tumor-promoting inflammation activity of miR-34a in colorectal cancer cell lines[J]. Mol Biol Rep, 2021, 48(1): 203-218. DOI: 10.1007/s11033-020-06035-9
[42] LUO Y, CHEN J J, LV Q, et al. Long non-coding RNA NEAT1 promotes colorectal cancer progression by competitively binding miR-34a with SIRT1 and enhancing the Wnt/beta-catenin signaling pathway[J]. Cancer Lett, 2019, 440-441: 11-22. DOI: 10.1016/j.canlet.2018.10.002
[43] IEKUSHI K, SEEGER F, ASSMUS B, et al. Regulation of cardiac microRNAs by bone marrow mononuclear cell therapy in myocardial infarction[J]. Circulation, 2012, 125(14): 1765-1773, S1-S7. DOI: 10.1161/CIRCULATIONAHA.111.079699
[44] BOON R A, IEKUSHI K, LECHNER S, et al. MicroRNA-34a regulates cardiac ageing and function[J]. Nature, 2013, 495(7439): 107-110. DOI: 10.1038/nature11919
[45] XIA W, CHEN H, CHEN D, et al. PD-1 inhibitor inducing exosomal miR-34a-5p expression mediates the cross talk between cardiomyocyte and macrophage in immune checkpoint inhibitor-related cardiac dysfunction[J]. J Immunother Cancer, 2020, 8(2): e001293. DOI: 10.1136/jitc-2020-001293
[46] XIA W, ZOU C, CHEN H, et al. Immune checkpoint inhibitor induces cardiac injury through polarizing macrophages via modulating microRNA-34a/Kruppel-like factor 4 signaling[J]. Cell Death Dis, 2020, 11(7): 575. DOI: 10.1038/s41419-020-02778-2
[47] HUANG Y, QI Y, DU J Q, et al. MicroRNA-34a regulates cardiac fibrosis after myocardial infarction by targeting Smad4[J]. Expert Opin Ther Targets, 2014, 18(12): 1355-1365.
[48] ZHANG C, ZHANG Y, ZHU H, et al. MiR-34a/miR-93 target c-Ski to modulate the proliferaton of rat cardiac fibroblasts and extracellular matrix deposition in vivo and in vitro[J]. Cell Signal, 2018, 46: 145-153. DOI: 10.1016/j.cellsig.2018.03.005
[49] SHI J, SURMA M, YANG Y, et al. Disruption of both ROCK1 and ROCK2 genes in cardiomyocytes promotes autophagy and reduces cardiac fibrosis during aging[J]. FASEB J, 2019, 33(6): 7348-7362. DOI: 10.1096/fj.201802510R
[50] ZHANG W C, YANG J H, LIU G H, et al. miR-34b/c regulates doxorubicin-induced myocardial cell injury through ITCH[J]. Cell Cycle, 2019, 18(23): 3263-3274. DOI: 10.1080/15384101.2019.1673618
[51] GIRICZ Z, MAKKOS A, SCHRECKENBERG R, et al. Swiprosin-1/EFhD-2 expression in cardiac remodeling and post-infarct repair: effect of ischemic conditioning[J]. Int J Mol Sci, 2020, 21(9): 3359. DOI: 10.3390/ijms21093359
[52] SCHULTHEISS H P, FAIRWEATHER D, CAFORIO A L P, et al. Dilated cardiomyopathy[J]. Nat Rev Dis Primers, 2019, 5(1): 32. DOI: 10.1038/s41572-019-0084-1
[53] ZHANG X L, ZHANG G, BAI Z H. miR-34a attenuates myocardial fibrosis in diabetic cardiomyopathy mice via targeting Pin-1[J]. Cell Biol Int, 2021, 45(3): 642-653. DOI: 10.1002/cbin.11512
[54] GATSIOU A, GEORGIOPOULOS G, VLACHOGIANNIS N I, et al. Additive contribution of microRNA-34a/b/c to human arterial ageing and atherosclerosis[J]. Atherosclerosis, 2021, 327: 49-58. DOI: 10.1016/j.atherosclerosis.2021.05.005
[55] WU J, LI X, LI D, et al. MicroRNA-34 family enhances wound inflammation by targeting LGR4[J]. J Invest Dermatol, 2020, 140(2): 465-476. e11. DOI: 10.1016/j.jid.2019.07.694
[56] NA J, LEE K, NA W, et al. Histone H3K27 demethylase JMJD3 in cooperation with NF-kappaB regulates keratinocyte wound healing[J]. J Invest Dermatol, 2016, 136(4): 847-858. DOI: 10.1016/j.jid.2015.11.029
[57] OSTRYCHARZ E, WASIK U, KEMPINSKA-PODHORODECKA A, et al. Melatonin protects cholangiocytes from oxidative stress-induced proapoptotic and proinflammatory stimuli via miR-132 and miR-34[J]. Int J Mol Sci, 2020, 21(24): 9667. DOI: 10.3390/ijms21249667
[58] CARRILLO-VICO A, LARDONE P J, ALVAREZ-SANCHEZ N, et al. Melatonin: buffering the immune system[J]. Int J Mol Sci, 2013, 14(4): 8638-8683. DOI: 10.3390/ijms14048638
[59] LI Y L, LIU X M, ZHANG C Y, et al. MicroRNA-34a/EGFR axis plays pivotal roles in lung tumorigenesis[J]. Oncogenesis, 2017, 6(8): e372. DOI: 10.1038/oncsis.2017.50
[60] ZHANG H, ZHANG X M, ZONG D D, et al. miR-34a-5p up-regulates the IL-1beta/COX2/PGE2 inflammation pathway and induces the release of CGRP via inhibition of SIRT1 in rat trigeminal ganglion neurons[J]. FEBS Open Bio, 2021, 11(1): 300-311. DOI: 10.1002/2211-5463.13027
[61] CHEN W, CAO G, YUAN X, et al. Notch-1 knockdown suppresses proliferation, migration and metastasis of salivary adenoid cystic carcinoma cells[J]. J Transl Med, 2015, 13: 167. DOI: 10.1186/s12967-015-0520-2
[62] LIU J J, ZHANG L, ZHANG F F, et al. Influence of miR-34a on preeclampsia through the Notch signaling pathway[J]. Eur Rev Med Pharmacol Sci, 2019, 23(3): 923-931.
[63] CHEVALIER B, ADAMIOK A, MERCEY O, et al. miR-34/449 control apical actin network formation during multiciliogenesis through small GTPase pathways[J]. Nat Commun, 2015, 6: 8386. DOI: 10.1038/ncomms9386
[64] ZHANG S, CUI Z. MicroRNA-34b-5p inhibits proliferation, stemness, migration and invasion of retinoblastoma cells via Notch signaling[J]. Exp Ther Med, 2021, 21(3): 255. DOI: 10.3892/etm.2021.9686
[65] CHEN Y, GAO D Y, HUANG L. In vivo delivery of miRNAs for cancer therapy: challenges and strategies[J]. Adv Drug Deliv Rev, 2015, 81: 128-141. DOI: 10.1016/j.addr.2014.05.009
[66] LIN F, WEN D, WANG X, et al. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer[J]. Biomaterials, 2019, 192: 95-108. DOI: 10.1016/j.biomaterials.2018.10.036
[67] SCHULZ A, MEYER F, DUBROVSKA A, et al. Cancer stem cells and radioresistance: DNA repair and beyond[J]. Cancers (Basel), 2019, 11(6): 862. DOI: 10.3390/cancers11060862
[68] PARK E Y, CHANG E, LEE E J, et al. Targeting of miR34a-NOTCH1 axis reduced breast cancer stemness and chemoresistance[J]. Cancer Res, 2014, 74(24): 7573-7582. DOI: 10.1158/0008-5472.CAN-14-1140
[69] VARES G, AHIRE V, SUNADA S, et al. A multimodal treatment of carbon ions irradiation, miRNA-34 and mTOR inhibitor specifically control high-grade chondrosarcoma cancer stem cells[J]. Radiother Oncol, 2020, 150: 253-261. DOI: 10.1016/j.radonc.2020.07.034
[70] HANAFI A R, JAYUSMAN A M, ALFASUNU S, et al. Serum MiRNA as predictive and prognosis biomarker in advanced stage non-small cell lung cancer in indonesia[J]. Zhongguo Fei Ai Za Zhi, 2020, 23(5): 321-332.
[71] AGOSTINI M, KNIGHT R A. miR-34: from bench to bedside[J]. Oncotarget, 2014, 5(4): 872-881. DOI: 10.18632/oncotarget.1825
[72] SESSA F, MAGLIETTA F, BERTOZZI G, et al. Human brain injury and miRNAs: an experimental study[J]. Int J Mol Sci, 2019, 20(7): 1546. DOI: 10.3390/ijms20071546
[73] BLANCO E, SHEN H, FERRARI M. Principles of nanoparticle design for overcoming biological barriers to drug delivery[J]. Nat Biotechnol, 2015, 33(9): 941-951. DOI: 10.1038/nbt.3330



 下载:
下载: