2. 复旦大学附属中山医院介入治疗科, 上海 200032;
3. 中山大学孙逸仙纪念医院胆胰外科, 广州 510220
2. Department of Interventional Radiology, Zhongshan Hospital, Fudan University, Shanghai 200032, China;
3. Department of Biliary and Pancreatic Surgery, Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University, Guangzhou 510220, Guangdong, China
胰腺癌恶性程度较高,5年生存率低于5%。因早期诊断率偏低,症状隐匿及缺乏有效的治疗方法,胰腺癌患者的生存率偏低。虽然围绕胰腺癌开展了较多研究,但仍疗效欠佳[1]。近年来,随着免疫治疗的兴起,针对T细胞和肿瘤相互作用的研究为肿瘤的免疫干预和治疗提供了理论基础。虽然免疫治疗在黑色素瘤、肺癌等实体瘤中免疫治疗效果显著,但针对胰腺癌却收效甚微[2]。导致胰腺癌免疫治疗效果欠佳的原因之一在于其独特的肿瘤微环境(tumor microenvironment, TME)。随着胰腺癌的发生发展,TME的组分和免疫状态不断发生变化,从而导致免疫效应细胞出现耗竭或重塑,促进肿瘤细胞发生免疫逃逸、侵袭转移及拮抗治疗等[3]。Tregs是胰腺癌TME中的主要组成成分,Tregs不仅能抑制抗肿瘤免疫的活性,还参与免疫微环境的重塑过程[4]。本文通过综述Tregs在胰腺癌免疫微环境重塑中的研究进展,从而寻求胰腺癌免疫治疗的新策略。
1 Tregs的概述、分类及其功能Tregs是一类发挥负性调节功能的CD4+ T细胞。正常情况下,Tregs通过维持机体免疫耐受能预防慢性炎症反应的发生。当肿瘤发生时,Tregs抑制机体的抗肿瘤反应导致肿瘤细胞发生免疫逃逸。叉头框转录因子3 (forkhead box P3, FoxP3)是叉头框转录因子家族成员,在人和小鼠体内作为Tregs的重要转录因子,FoxP3对Tregs的发育成熟及Tregs介导的肿瘤免疫至关重要。FoxP3特异表达于Tregs中,是TME中Tregs的有效标志物[5]。
Tregs主要分为胸腺来源的Tregs (thymic Tregs, tTregs),由普通T细胞在体外诱导分化的体外诱导Tregs (induced Tregs, iTregs) 和外周组织中由抗原刺激T细胞分化而来的内源性诱导Tregs (peripheral Tregs, pTregs)[6]。其中tTregs也被称为天然型Tregs(natural Tregs, nTregs)。nTregs主要由骨髓中的祖细胞在胸腺中分化形成。nTregs能持续表达FoxP3并且通过细胞接触作用在机体炎症部位及肿瘤组织中发挥免疫抑制作用。iTregs分化则需要有足够的同源抗原及免疫调节因子如转化生长因子-β(transforming growth factor-β, TGF-β)、白介素2 (IL-2)、IL-10、干扰素-γ(IFN-γ)进行诱导[7-9]。在TME中,nTregs与iTregs的表型及作用可以发生相互转化[10]。Tregs能通过抑制性细胞因子IL-10、IL-35、TGF-β、免疫检查点和程序性死亡受体1 (programmed cell death protein 1, PD-1)、淋巴细胞活化基因3 (lymphocyte-activation gene 3, LAG-3)、细胞毒性T淋巴细胞相关蛋白4 (cytotoxic lymphocyte antigen 4, CTLA-4)、T细胞免疫球蛋白及黏蛋白结构域分子3 (T cell immunoglobulin domain and mucin domain 3, TIM-3)、免疫球蛋白和免疫球蛋白结构域的T细胞免疫受体(T cell immunoreceptor with Ig and ITIM domains, TIgIT), 促进T细胞衰竭与扩增的耐受性树突状细胞及穿孔素/颗粒酶介导的细胞毒性等发挥其抑制作用[11-12]。FoxP3+ Tregs在TME中高表达可能提示预后不良,然而在肿瘤内出现Tregs细胞高浸润缺乏显著提示作用,甚至在结直肠癌患者肿瘤组织中,Tregs细胞高密度浸润提示患者预后较好,这可能和肿瘤类型及Tregs细胞的定位分布等有关[10, 13]。
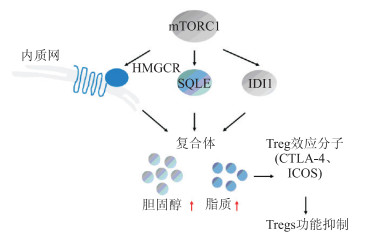
2 TME的能量代谢对Tregs的影响细胞代谢在Tregs分化和正常功能行使中发挥关键作用。Tregs可以利用替代底物和代谢途径获取能量。雷帕霉素靶蛋白(mTOR)复合物能感知周围的营养物及生长因子的细微变化,从而调节Tregs的分化及功能状态。mTORC1能诱导3-羟基-3-甲基戊二酰-CoA还原酶(anti-hydroxy-3-methylglutaryl-CoA reductase, HMGCR)、角鲨烯环氧酶(squalene epoxidase, SQLE)及异戊二磷酸异构酶1 (isopentenyl-diphosphate isomerase 1, IDI1),从而驱动胆固醇和脂质的生物合成,这些对Tregs标志物CTLA-4和T细胞可诱导共刺激分子(inducible T-cell costimulator,ICOS)的表达十分重要[14-15](图 1)。
|
| 图 1 mTORC1参与维持Tregs在TME中的免疫抑制功能 mTORC1:mTOR复合物1;HMGCR:3-羟基-3-甲基戊二酰-CoA还原酶;SQLE:角鲨烯环氧酶;IDI1:异戊二磷酸异构体1;CTLA-4:细胞毒性T淋巴细胞相关蛋白4;ICOS:T细胞可诱导共刺激分子;TME:肿瘤微环境。 |
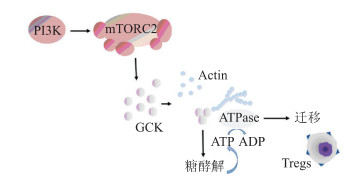
葡萄糖激酶介导的糖酵解对于Tregs的迁移也发挥重要作用。Kishore等[16]发现,Tregs的迁移需要通过磷脂酰肌醇3-激酶(PI3K)-mTORC2途径诱导的葡萄糖激酶激活糖酵解完成。缺乏该途径的Tregs虽然具有免疫抑制作用,但不能迁移到同种异体皮肤移植物中抑制排斥反应(图 2)。PI3K信号的调节也可改变细胞代谢和FoxP3的表达,同源性磷酸酶-张力蛋白(phosphatase and tensin homolog, PTEN) 作为PI3K的主要负调节因子能抑制糖酵解活性。机体通过PTEN调控PI3K,限制蛋白激酶B (protein kinase B, AKT)磷酵化,进而维持Tregs稳态和功能[17] (图 3)。TME中特定代谢物的存在也同样影响Tregs的抑制功能及谱系的稳定性。例如,吲哚胺2,3-双加氧酶(indoleamine 2, 3-dioxygenase, IDO)产生的色氨酸代谢产物犬尿氨酸作为一种激芳基烃受体(aryl hydrocarbon receptor, AHR)激动剂对维持Tregs的抑制功能具有重要意义[18]。另外,与维生素A、D等相关的生物活性代谢产物同样能影响Tregs的细胞功能。维生素A的生物活性代谢产物维甲酸(retinoic acid, RA)能促进幼稚T细胞转化为FoxP3+ Tregs。维生素D的活性代谢产物钙三醇同样也能促进FoxP3+ Tregs生长[19-20]。
|
| 图 2 糖酵解有助于Tregs的迁移 PI3K:磷脂酰肌醇3-激酶;mTORC2:mTOR蛋白复合体2;GCK:葡萄糖激酶。 |

|
| 图 3 PTEN通过抑制AKT的磷酸化维持Tregs的稳定性 PTEN:同源性磷酸酶-张力蛋白;PI3K:磷脂酰肌醇3-激酶。 |
Tregs能抑制机体对肿瘤细胞发生免疫应答,从而给肿瘤细胞提供适宜的生长条件,并且肿瘤细胞也能主动诱导Tregs的迁移、分化及扩增,从而抑制针对肿瘤相关抗原(tumor associated antigen, TAA)的自发性T细胞免疫及一系列的抗肿瘤免疫反应。在肿瘤免疫微环境中,Tregs主要通过直接接触抑制及间接接触抑制发挥作用。
在直接接触抑制中,经过T细胞抗原受体(T cell receptor, TCR)途径激活的Tregs细胞膜上大量表达TGF-β,TGF-β通过与靶细胞上的受体相结合而抑制效应B细胞和T细胞的功能。另外,在直接接触抑制中,活化的Tregs上也能表达共抑制分子CTLA-4。在正常情况下,T细胞的活化需要TCR与抗原递呈细胞(antigen presenting cell, APC)递呈的MHC-抗原肽复合物相互结合及B7分子与T细胞表面的CD28相结合这两条信号通路共同激活。激活的Tregs上高表达的CTLA-4与CD28具有较高同源性但是功能相反,因此Tregs上的CTLA-4能与B7分子结合,从而抑制T细胞的活化。
在间接接触抑制中,Tregs能通过分泌IL-10、IL-35、TGF-β及纤维蛋白原样蛋白2 (fibrinogen-like protein 2, FGL2)等细胞因子参与免疫抑制。TGF-β能通过抑制CD8+T的细胞毒作用介导胰腺癌细胞发生免疫逃逸。在长期生存的胰腺癌患者中,肿瘤浸润的CD8+T细胞数量明显增加[21-24]。Cheng等[25]研究发现,胰腺癌中KrasG12D突变也能促进CD4+T细胞向Tregs转化,通过调控MEK/ERK通路促进ERK磷酸化,从而使TGF-β和IL-10表达上调,进一步富集Tregs造成TME的免疫抑制。Tregs除直接发挥免疫微环境重塑作用外,还能与骨髓来源抑制性细胞(myeloid-derived suppressor cell, MDSC)及肿瘤相关巨噬细胞(tumor-associated macrophage, TAM)协同分泌免疫抑制因子,抑制CD8+T淋巴细胞的抗肿瘤作用,间接导致免疫细胞数量和功能失衡,从而形成胰腺癌特殊的免疫抑制TME[26-27]。
4 Tregs在胰腺癌中的临床意义在不同级别的胰腺导管上皮内瘤变和胰腺导管腺癌组织中,Tregs在胰腺癌癌前病变的演变过程中数量逐渐增加,并且Tregs的数量与胰腺癌肿瘤细胞转移、肿瘤分级及分期相关[28]。Tregs在胰腺导管腺癌癌前病变至癌变期间的免疫反应过程中发挥重要调控作用。Tregs数量增多通常提示胰腺癌患者预后不良。在胰腺癌肿瘤组织中,Tregs、CD4+ T及CD8+ T细胞的浸润百分比显著高于周围正常的胰腺组织。Tregs能抑制局部肿瘤内CD8+T细胞的抗肿瘤免疫,从而促进胰腺导管腺癌的发展;胰腺癌肿瘤组织中Tregs数目的增加也提示机体内出现了被抑制的抗肿瘤免疫反应[29]。与健康人群相比,胰腺癌患者外周血中的Tregs数量明显增加,并且与胰腺癌TNM分期相关。大部分T1/T2分期的胰腺癌患者Tregs数目少于晚期患者,并且Tregs数目越少的患者出现淋巴结转移的概率越小。另外,吉西他滨、环磷酰胺和紫杉烷类药物联用能显著降低胰腺癌患者TME中Tregs的数目[30]。在进行胰腺癌根治切除手术后的患者中,Tregs数量增加通常提示生存期缩短。在接受化疗的胰腺癌患者中,处于疾病稳定期和部分缓解期的患者Tregs水平显著降低,处于进展期患者的Tregs水平则出现升高。在经过2个周期的化疗后,Tregs细胞数目或CD4/CD8比值降低提示患者有更长的生存期。在接受新辅助治疗的胰腺癌患者中,Tregs数目降低将有利于局部肿瘤组织的抗肿瘤免疫反应。Tregs数目的相对增加可能与机体的免疫抑制及进展有关,而Tregs的免疫学监视有助于预测胰腺癌患者的预后[31]。
5 靶向Tregs治疗胰腺癌的新策略基于Tregs细胞在TME中的特点,减少Tregs细胞浸润的数量,降低其免疫抑制作用有助于探索胰腺癌的治疗新策略。目前,多采用环磷酰胺、抗CTLA-4单抗、CD25单抗等清除Tregs细胞的浸润。
CTLA-4在激活的T淋巴细胞和Tregs细胞上表达较高,利用抗CTLA-4单抗能诱导Tregs细胞凋亡,降低免疫抑制作用。一些抗CTLA-4单抗(如L3D10及Ipilimumab/HL12/HL32)能有效降低肿瘤局部的Tregs细胞数目。其中Ipilimumab的Ⅱ期临床试验表明CTLA-4抗体在黑色素瘤中具有较好的疗效,但同时也导致不同程度免疫不良反应[32-33]。而在胰腺癌动物模型中,在肿瘤局部应用低剂量抗CTLA-4单抗能持续产生抗肿瘤效应。这一发现为超声引导下的胰腺癌瘤内抗CTLA-4抗体注射方案提供了理论基础[34]。
应用低剂量的环磷酰胺也能减少Tregs细胞的数量,并且能抑制Tregs细胞的功能并降低其增殖能力。在注射基因转导的自体肿瘤疫苗(gene transduced autologous tumor vaccine,GVAX)前24 h给予胰腺癌患者一定剂量的环磷酰胺能减少Tregs细胞的数目增强肿瘤疫苗的功效[35]。
IL-2α的受体CD25也是Tregs细胞的一个重要干预靶点,其与IL-2α的亲和力及细胞功能受到严格调控。CD25在激活的循环免疫细胞及Tregs细胞表面高表达。在小鼠模型中联合阻断CD25和TGF-β能明显减少周围组织和肿瘤组织内的FoxP3+ Tregs细胞的浸润数目[36]。在胰腺癌Pan02细胞系小鼠移植瘤模型实验中,联合使用CD25单克隆抗体、toll样受体9 (toll-like receptor 9,TLR9)激动剂CpG和胰腺癌细胞特异性抗原免疫激活复合物(immune stimulatory complexes,ISCOM)疫苗能降低肿瘤组织中Tregs细胞的浸润进而抑制肿瘤的生长[37]。另外,在小鼠胰腺癌模型中,树突状细胞(dendritic cell, DC)与肿瘤细胞(tumor cell, TC)融合疫苗联合CD25单克隆抗体能提高NK细胞的活性。当耗竭一部分Tregs后,小鼠体内细胞毒性T细胞的活性显著增强,淋巴细胞IFN-γ的分泌能力也明显提高。这些结果都提示Tregs耗竭联合DC/TC融合疫苗能提高胰腺癌的免疫治疗效果[38]。
另外,一些信号通路在维持Tregs细胞功能上也发挥一定作用。激活PI3K-AKT-mTOR信号通路能抑制Tregs细胞分化。PI3K-AKT-mTOR信号通路在Tregs细胞的生长分化中发挥重要作用;在小鼠体内抑制PI3K-AKT-mTOR信号通路会使Tregs细胞特异性基因FoxP3表达增强,从而导致Tregs细胞数量增加。利用PI3K抑制剂LY294002及mTOR抑制剂雷帕霉素抑制PI3K-AKT-mTOR信号通路能诱导活化的CD4+ T细胞表达FoxP3[39-40]。
6 小结Tregs细胞在肿瘤免疫逃逸、肿瘤免疫微环境重塑中发挥重要作用。动态监测肿瘤患者机体内Tregs细胞的数目变化对判断肿瘤发生发展、评估治疗效果及预后具有重要意义,但因肿瘤的异质性,借助Tregs指导胰腺癌个体化治疗,根据个体患者制定联合治疗方案推动精准治疗将面临挑战。目前,针对Tregs的免疫治疗也尚未成熟,作为维持人体免疫稳态的细胞群之一,清除Tregs可能会导致患者出现严重的自身免疫并发症,在治疗过程中如何针对TME中的Tregs而不影响机体的免疫稳态也是亟需解决的关键问题。
利益冲突:所有作者声明不存在利益冲突。
| [1] |
WEI L, WEN J Y, CHEN J, et al. Oncogenic ADAM28 induces gemcitabine resistance and predicts a poor prognosis in pancreatic cancer[J]. World J Gastroenterol, 2019, 25(37): 5590-5603.
[DOI]
|
| [2] |
VAN DEN BULK J, VERDEGAAL E M, DE MIRANDA N F, et al. Cancer immunotherapy: broadening the scope of targetable tumours[J]. Open Biol, 2018, 8(6): 180037.
[DOI]
|
| [3] |
HO W J, JAFFEE E M, ZHENG L, et al. The tumour microenvironment in pancreatic cancer-clinical challenges and opportunities[J]. Nat Rev Clin Oncol, 2020, 17(9): 527-540.
[DOI]
|
| [4] |
ZHANG Y, LAZARUS J, STEELE N G, et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis[J]. Cancer Discov, 2020, 10(3): 422-439.
[DOI]
|
| [5] |
SALEH R, ELKORD E. Foxp3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets[J]. Cancer Lett, 2020, 490: 174-185.
[DOI]
|
| [6] |
SHEVACH E M, THORNTON A M. tTregs, pTregs, and iTregs: similarities and differences[J]. Immunol Rev, 2014, 259(1): 88-102.
[DOI]
|
| [7] |
KANAMORI M, NAKATSUKASA H, OKADA M, et al. Induced regulatory T cells: their development, stability, and applications[J]. Trends Immunol, 2016, 37(11): 803-811.
[DOI]
|
| [8] |
PALUSKIEVICZ C M, CAO X, ABDI R, et al. T regulatory cells and priming the suppressive tumor microenvironment[J]. Front Immunol, 2019, 10: 2453.
[DOI]
|
| [9] |
ZHANG Q, FU L, LIANG Y, et al. Exosomes originating from MSCs stimulated with TGF-β and IFN-γ promote Treg differentiation[J]. J Cell Physiol, 2018, 233(9): 6832-6840.
[DOI]
|
| [10] |
BELAL CHAUDHARY, EYAD ELKORD. Regulatory T Cells in the tumor microenvironment and cancer progression: role and therapeutic targeting[J]. Vaccines, 2016, 4(3): 28.
[DOI]
|
| [11] |
SCHMIDT A, OBERLE N, KRAMMER P H. Molecular mechanisms of treg-mediated T cell suppression[J]. Front Immunol, 2012, 3: 51.
|
| [12] |
SASIDHARAN NAIR V, ELKORD E. Immune checkpoint inhibitors in cancer therapy: a focus on T-regulatory cells[J]. Immunol Cell Biol, 2018, 96(1): 21-33.
[DOI]
|
| [13] |
NICOLAY JP, ALBRECHT JD, ALBERTI-VIOLETTI S, et al. CCR4 in cutaneous T-cell lymphoma: therapeutic targeting of a pathogenic driver[J]. Eur J Immunol, 2021, 51(7): 1660-1671.
[DOI]
|
| [14] |
SHI G, LI D, REN J, et al. mTOR inhibitor INK128 attenuates dextran sodium sulfate-induced colitis by promotion of MDSCs on Treg cell expansion[J]. J Cell Physiol, 2019, 234(2): 1618-1629.
[DOI]
|
| [15] |
ZENG H, YANG K, CLOER C, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function[J]. Nature, 2013, 499(7459): 485-490.
[DOI]
|
| [16] |
KISHORE M, CHEUNG K C P, FU H, et al. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis[J]. Immunity, 2017, 47(5): 875-889.
[DOI]
|
| [17] |
CETINTAS V B, BATADA N N. Is there a causal link between PTEN deficient tumors and immunosuppressive tumor microenvironment?[J]. J Transl Med, 2020, 18: 45.
[DOI]
|
| [18] |
CAMPESATO L F, BUDHU S, TCHAICHA J, et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine[J]. Nat Commun, 2020, 11(1): 4011.
[DOI]
|
| [19] |
RAVERDEAU M, MILLS K H. Modulation of T cell and innate immune responses by retinoic Acid[J]. J Immunol, 2014, 192(7): 2953-2958.
[DOI]
|
| [20] |
VANHERWEGEN A S, EELEN G, FERREIRA G B, et al. Vitamin D controls the capacity of human dendritic cells to induce functional regulatory T cells by regulation of glucose metabolism[J]. J Steroid Biochem Mol Biol, 2019, 187: 134-145.
[DOI]
|
| [21] |
OVCINNIKOVS V, ROSS E M, PETERSONE L, et al. CTLA-4-mediated transendocytosis of costimulatory molecules primarily targets migratory dendritic cells[J]. Sci Immunol, 2019, 4(35): eaaw0902.
[DOI]
|
| [22] |
BALACHANDRAN V P, ŁUKSZA M, ZHAO J N, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer[J]. Nature, 2017, 551(7681): 512-516.
[DOI]
|
| [23] |
DE STREEL G, BERTRAND C, CHALON N, et al. Selective inhibition of TGF-β1 produced by GARP-expressing Tregs overcomes resistance to PD-1/PD-L1 blockade in cancer[J]. Nat Commun, 2020, 11(1): 4545.
[DOI]
|
| [24] |
ULBAR F, VILLANOVA I, GIANCOLA R, et al. Clinical-grade expanded regulatory T cells are enriched with highly suppressive cells producing IL-10, granzyme B, and IL-35[J]. Biol Blood Marrow Transplant, 2020, 26(12): 2204-2210.
[DOI]
|
| [25] |
CHENG H, FAN K, LUO G, et al. KrasG12D mutation contributes to regulatory T cell conversion through activation of the MEK/ERK pathway in pancreatic cancer[J]. Cancer Lett, 2019, 446: 103-111.
[DOI]
|
| [26] |
SIRET C, COLLIGNON A, SILVY F, et al. Deciphering the crosstalk between myeloid-derived suppressor cells and regulatory T cells in pancreatic ductal adenocarcinoma[J]. Front Immunol, 2020, 10: 3070.
[DOI]
|
| [27] |
BOISSONNAS A, LICATA F, POUPEL L, et al. CD8+ tumor-infiltrating T cells are trapped in the tumor-dendritic cell network[J]. Neoplasia, 2013, 15(1): 85-94.
[DOI]
|
| [28] |
HIRAOKA N, ONOZATO K, KOSUGE T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions[J]. Clin Cancer Res, 2006, 12(18): 5423-5434.
[DOI]
|
| [29] |
Group Young Researchers In Inflammatory Carcinogenesis, WANDMACHER A M, MEHDORN A S, et al. The heterogeneity of the tumor microenvironment as essential determinant of development, progression and therapy response of pancreatic cancer[J]. Cancers (Basel), 2021, 13(19): 4932.
[DOI]
|
| [30] |
ZHENG Y, DOU Y, DUAN L, et al. Using chemo-drugs or irradiation to break immune tolerance and facilitate immunotherapy in solid cancer[J]. Cell Immunol, 2015, 294(1): 54-59.
[DOI]
|
| [31] |
HERTING C J, KARPOVSKY I, LESINSKI G B, et al. The tumor microenvironment in pancreatic ductal adenocarcinoma: current perspectives and future directions[J]. Cancer Metastasis Rev, 2021, 40(3): 675-689.
[DOI]
|
| [32] |
DU X, TANG F, LIU M, et al. A reappraisal of CTLA-4 checkpoint blockade in cancer immunotherapy[J]. Cell Res, 2018, 28(4): 416-432.
[DOI]
|
| [33] |
LARKIN J, CHIARION-SILENI V, GONZALEZ R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma[J]. N Engl J Med, 2015, 373(1): 23-34.
[DOI]
|
| [34] |
SANDIN L C, ERIKSSON F, ELLMARK P, et al. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo[J]. Oncoimmunology, 2014, 3(1): e27614.
[DOI]
|
| [35] |
MAYOR S. Immunotherapy improves overall survival in pancreatic cancer[J]. Lancet Oncol, 2015, 16(2): e58.
|
| [36] |
PU N, ZHAO G, YIN H, et al. CD25 and TGF-β blockade based on predictive integrated immune ratio inhibits tumor growth in pancreatic cancer[J]. J Transl Med, 2018, 16(1): 294.
[DOI]
|
| [37] |
JACOBS C, DUEWELL P, HECKELSMILLER K, et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma[J]. Int J Cancer, 2011, 128(4): 897-907.
[DOI]
|
| [38] |
YAMAMOTO M, KAMIGAKI T, YAMASHITA K, et al. Enhancement of anti-tumor immunity by high levels of Th1 and Th17 with a combination of dendritic cell fusion hybrids and regulatory T cell depletion in pancreatic cancer[J]. Oncol Rep, 2009, 22(2): 337-343.
|
| [39] |
FAN MY, TURKA LA. Immunometabolism and PI(3)K signaling as a link between IL-2, Foxp3 expression, and suppressor function in regulatory T cells[J]. Front Immunol, 2018, 9: 69.
[DOI]
|
| [40] |
YATES J, ROVIS F, MITCHELL P, et al. The maintenance of human CD4+ CD25+ regulatory T cell function: IL-2, IL-4, IL-7 and IL-15 preserve optimal suppressive potency in vitro[J]. Int Immunol, 2007, 19(6): 785-799.
[DOI]
|
 2022, Vol. 29
2022, Vol. 29


