近年来,糖生物学在癌症中的研究越发深入。聚糖通过多种途径调控肿瘤微环境中细胞的结构与功能,在肿瘤发生、发展过程中发挥重要的调节作用,为肿瘤的诊断和治疗提供一系列独特靶点[1-2]。聚糖具有调控肿瘤微环境中免疫细胞的功能,作用机制为以蛋白质糖基化形式改变细胞黏附分子及其配体表达,下调免疫细胞的抗肿瘤免疫功能,促进肿瘤免疫逃逸和转移[3-4]。糖基化的研究[5-6]发现,程序性细胞死亡受体1(PD1)、程序性细胞死亡配体1(PD-L1)等免疫检查点分子的糖基化会明显抑制免疫细胞的抗肿瘤免疫作用。此外,免疫检查点去糖基化治疗能够再次恢复免疫细胞的肿瘤抗性并提升细胞毒性化学治疗药物的疗效[5]。本文就糖基化修饰对于肿瘤免疫的影响予以综述。
1 糖基化糖基化是一种重要的蛋白质翻译后修饰,是在糖基转移酶的控制下,将糖键结合至蛋白质或脂质上形成糖苷键的过程。蛋白质经过糖基化作用,形成糖蛋白,聚糖通过氮或氧基连接在多肽链上,可以分为N-聚糖和O-聚糖(图 1)。糖缀合物根据其苷元部分的性质和连接进行定义,主要包括蛋白聚糖和鞘糖脂。蛋白聚糖是含有一种或多种糖胺聚糖(glycosaminoglycan, GAG)的糖缀合物,如硫酸角蛋白、硫酸软骨素和硫酸肝素等[7]。
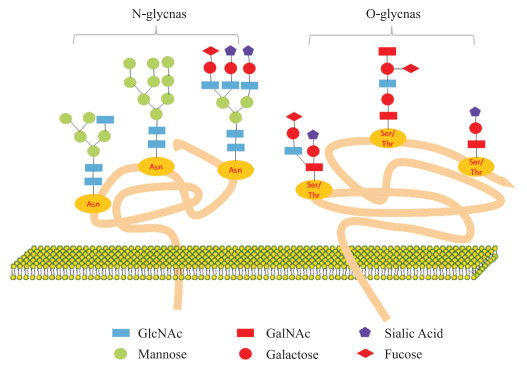
|
| 图 1 N/O-糖基化示意图 Fig 1 N/O-glycosylation schematic diagram N-Glycosylation involves the attachment of GlcNAc to the nitrogen atom of certain asparagine side-chain amide groups within the peptide chain (the connection must occur between asparagine-any amino acid other than proline-serine/threonine residues). O-Glycosylation primarily occurs through the connection of GalNAc to the hydroxyl oxygen atom of serine/threonine residues within the peptide chain. Asn: Asparagine; Ser: Serine; Thr: Threonine. |
N-糖基化以长萜醇为聚糖载体,起始于内质网的天冬酰胺-X-丝氨酸/苏氨酸(X是除脯氨酸以外的任何氨基酸)结构,在天冬氨酸残基端形成包含2个乙酰葡糖胺(N-acetylglucosamine, GlcNAc),9个甘露糖和3个葡萄糖的前体14糖链结构。初步N-糖基化后寡糖连接的蛋白质序列可以正确折叠,并被转运到高尔基体去除甘露糖残基。
O-糖基化也属于一种频繁的蛋白质翻译后修饰模式,但不同于N-糖基化,该糖基化过程主要发生在高尔基体。O-糖链结构简单且较短,种类比N-糖链丰富。肽链中发生O-糖基化部位的主要为丝氨酸和苏氨酸残基,此外还包括酪氨酸、羟赖氨酸和羟脯氨酸,连接的位点为上述氨基酸侧链上的羟基。O-连接聚糖常以N-乙酰半乳糖胺(N-acetylgalactosamine, GalNAc)和半乳糖构成核心二糖。有趣的是,O-糖基化修饰和磷酸化修饰作用方式相近,两者相互影响共同调节生物体的生命活动[8-9]。
2 肿瘤微环境与糖基化与正常细胞相比,肿瘤细胞存在更广泛的蛋白糖基化[10],该现象增加肿瘤细胞群内的分子异质性和功能多样性。Hakomori等[11]首先提出肿瘤微环境糖基化结构改变的2个概念:不完全合成和新合成。不完全合成即在肿瘤发生早期,因细胞损伤导致聚糖在正常细胞上错误合成;新合成则发生在肿瘤晚期,在肿瘤相关的基因诱导下发生异常糖基化。
为解释肿瘤细胞影响微环境并改变微环境中细胞正常糖基化途径这一现象,科学家们提出几种可能的原因:(1)聚糖表达的改变可能源于可变受体底物的可变性、糖核苷酸供体和辅助因子的可用性与丰度差异[12]。(2)聚糖表达的改变可归因于糖基转移酶的不足或过量。与其转录水平的调节紊乱,伴侣蛋白功能的失调以及糖苷酶活性的改变相关[13-14]。(3)聚糖表达的改变可能是由于主肽链和新生聚糖链的三级构象改变,影响了糖基化修饰位点与修饰稳定性。(4)聚糖表达的改变可能是由于高尔基体中相关糖基转移酶的表达和定位发生改变所致。糖基转移酶的错误定位会导致免疫球蛋白酶核心聚糖结构的合成错误[15]。
肿瘤微环境中的糖基化水平广泛增高,导致受体酪氨酸激酶磷酸化受阻[16]、黏附分子发生异常糖基化[3, 17]等现象,继发细胞信号转导异常、细胞间黏附力降低,引起肿瘤细胞增殖和转移失控。
E-钙黏蛋白是一种跨膜糖蛋白,充当上皮细胞-细胞黏附的标志[18]。然而在肿瘤微环境中,N-乙酰氨基葡萄糖转移酶Ⅴ(GnT-Ⅴ)过表达可以增加E-钙黏蛋白的N-糖基化,导致蛋白组装错误并表现为无功能性的黏附连接,破坏细胞-细胞黏附表型,造成肿瘤的侵袭和转移[19]。与此相反,N-乙酰氨基葡萄糖转移酶Ⅲ对于E-钙黏蛋白的糖基化修饰与抑制细胞内吞作用,细胞膜的延迟周转率有关,并增加了黏附的稳定性,从而起到抑制肿瘤的作用[20]。
硫酸肝素蛋白聚糖(heparan sulfate proteogly-cans, HSPG)分布于细胞表面以及细胞外基质中,主要负责细胞的生长与分化,调控血管生成与稳定[21]。肿瘤微环境中蛋白的HSPG修饰增多时,可以激活例如EGFR、MET、TGFβ等分子,从而调节各种信号分子交互作用,促进肿瘤生长及运动信号转导[22-23]。此外,HSPG修饰的蛋白往往会释放内皮细胞生长因子,促进肿瘤微环境中微血管的生成及稳定,为肿瘤生长提供必要的营养及代谢条件。
基于肿瘤微环境的Warburg效应,葡萄糖经过己糖胺生物合成途径生成尿苷二磷酸-GlcNAc,随后被O-GlcNAc转移酶(O-GlcNAc transferase, OGT)调控,被用于O-GlcNAc修饰[24-25]。O-GlcNAc已被证明与磷酸化有广泛的交互,改变蛋白降解,并可以作为营养传感器来调节信号转导[26]。在MYC和p53蛋白上,O-GlcNAc修饰与磷酸化修饰竞争,动态调节MYC和p53蛋白的稳定性及活性,分别发挥促癌和抑癌作用[27-28]。
此外,GAGs也会随着整体糖代谢的改变在肿瘤微环境中富集,肿瘤细胞利用这些糖信号塑造免疫逃逸体系,影响细胞外基质组成,改变肿瘤细胞发生、发展、侵袭、转移和干细胞生态位维持等恶性表型[29]。
3 唾液酸聚糖与抗肿瘤免疫肿瘤微环境中的聚糖干扰肿瘤细胞对于免疫细胞的编辑,异常调控病原体识别相关的免疫过程及适应性免疫反应过程[30]。哺乳动物细胞表面的聚糖通常以唾液酸终止,这些唾液酸化的结构也被称为唾液酸聚糖,可以结合各种内源性受体,其中与肿瘤免疫关系最为密切的是唾液酸结合的免疫球蛋白样凝集素(siglecs)[31]。
人类siglecs亚家族包括siglecs-3、5、6、7、8、9、10和11[32]。其受体主要表达在免疫细胞上,肿瘤微环境唾液聚糖密度的增加可募集免疫细胞表面的抑制性siglecs受体,下调机体对肿瘤细胞的免疫反应[33]。也有研究[34]表明,siglecs受体通过与siglecs结合实现对活性氧的正负调控,进而下调或上调免疫功能。Siglecs-9与免疫细胞结合的同时能与肿瘤抗原MUC1黏蛋白结合,诱导连环蛋白的募集和随后的肿瘤细胞生长,导致巨噬细胞M2型极化产生抑制抗癌免疫的细胞因子和促血管生成因子,增强肿瘤细胞的侵袭从而促进肿瘤进展[35]。Siglec-9上调与siglec-E缺陷具有相同的巨噬细胞调控模式,在小鼠中发现siglec-E缺陷的巨噬细胞倾向于极化到M2巨噬细胞[36]。此外,siglecs-9表达于人类自然杀伤(natural killer, NK)细胞,抑制NK细胞介导的体外肿瘤杀伤,显著降低NK细胞对缺乏MHC1表达的肿瘤细胞的杀伤作用,降低抗体依赖的细胞毒性[37-38]。更有趣的是,siglecs受体类似于免疫检查点,作为潜在的“不吃我”信号抑制巨噬细胞介导的吞噬作用,动物模型的治疗上也呈现相似的疗效趋势。最近研究[39]发现,用单克隆抗体阻断唾液聚糖-siglec糖免疫检查点有利于肿瘤浸润淋巴细胞(tumor-infiltrating lymphocytes, TILs)的激活,特别是细胞毒性CD8+T细胞的激活,它是杀伤肿瘤细胞的主力军。对比肿瘤患者和健康人群,在非小细胞肺癌、结直肠癌、上皮性卵巢癌和黑素瘤患者中均发现siglecs受体上调,主要是siglec-9受体[40-41]。然而,健康的外周血T细胞并不表达如前面所描述的抑制性受体,表明siglecs是一个潜在改善抗肿瘤T细胞活化的可靠干预靶点。
4 免疫检查点糖基化免疫检查点是免疫系统中的抑制性通路,通过配体和受体相互作用发挥调控,对于维持自身免疫耐受、调节生理性免疫应答的持续时间和幅度起重要作用,避免免疫系统对正常组织造成损伤和破坏[42]。20世纪免疫检查点的发现是肿瘤免疫治疗的里程碑事件,随后几十年中免疫检查点抑制剂(immune checkpoint inhibitor, ICI)的治疗造福数量庞大的肿瘤及自身免疫病患者。2011年,抗细胞毒性T淋巴细胞抗原4抗体伊匹单抗(Ipilimumab)成为全球首个FDA批准的癌症免疫检查点阻断药物。此后一系列ICI获批上市用于抑制PD1及其配体PD-L1介导的通路[42-43]。ICI联合使用或者ICI联合化疗是多种晚期肿瘤的一线治疗方案,有效延长肿瘤患者的生存期。但是临床上由于患者的肿瘤突变负荷、肿瘤免疫微环境[44]和基因突变状态的差异化,导致免疫治疗效果参差不齐,且多种ICI联合治疗会伴随严重的并发症[45]。除了寻找新的免疫检查点,开发新的针对性抗体外,近10年免疫检查点相关研究逐渐把矛头指向了其糖基化带来的影响。
4.1 PD-1的糖基化PD-1主要表达于活化的T细胞或者B细胞表面。在正常生理状态下,PD-1激活主要发挥抑制过激炎症反应,起到预防自身免疫性疾病发生的作用[46-47]。在肿瘤微环境中,PD-1结合肿瘤细胞高表达的PD-L1,抑制T细胞的免疫功能或者耗竭T细胞数量,促进肿瘤免疫逃逸。研究[48]报道,PD-1存在4个N-糖基化位点,其中任何位点的去糖基化均下调PD-1蛋白表达水平。Sun等[6]发现,与FDA批准的PD-1单抗相比较,靶向PD-1 N58糖基化位点的单克隆抗体STM418亲和力更高,抗肿瘤活性更强。Wang等[49]构建的PD-1特异性单克隆抗体MW11-h317也显示出PD-1高度亲和力,对该单抗与PD-1的结合位点进行分析发现,其针对的主要也是PD-1的N58糖基化位点。然而近年引进的PD-1单抗西米普利单抗(Cemiplimab)呈现与其他PD-1单克隆抗体相反的结合机制。Cemiplimab/PD-1复合物的结构分析显示,Cemiplimab主要通过其重链与PD-1结合,而轻链则是PD-L1与PD-1结合竞争的主要区域[50]。该结合形式与卡瑞利珠单抗(Camrelizumab)和PD-1的相互作用网络相似[51]。进一步研究[50]发现,PD-1的N58聚糖参与PD-1与Cemiplimab的相互作用,且N58-聚糖缺陷型PD-1显著降低Cemiplimab与PD-1的结合亲和力,而与N58-糖基化缺陷型PD-1结合后,Cemiplimab的PD-1/PD-L1阻断效率降低。以上的研究结果证明,调控PD-1糖基化是提升PD-1免疫治疗疗效的新手段,但是需要根据不同的抗体特性来设计糖基化干扰计划。
4.2 PD-L1糖基化PD-L1可在肿瘤细胞表面表达,与活化T细胞表面PD-1结合,从而抑制T细胞的增殖、活化、迁移和细胞毒性作用。在肿瘤组织中,PD-L1也表现为高度N-糖基化,其糖基化修饰被认为进一步抑制T细胞的活性[5, 52]。肿瘤微环境中PD-L1的高度糖基化导致其免疫组织化学染色结果与患者免疫治疗疗效不一致[53],通过去糖基化酶处理后的肿瘤细胞显示出更高的PD-L1信号强度以及更强的PD-L1单抗亲和力[54]。Li等[55]发现三阴性乳腺癌中表皮生长因子诱导的PD-1和PD-L1结合需要β-1, 3-N-乙酰氨基葡萄糖转移酶-3的表达。更有意思的是在三阴性乳腺癌中,D-甘露糖和二甲双胍可以激活单磷酸腺苷蛋白激酶(adenosine-5'-monophosphate activated protein kinase, AMPK)诱导PD-L1蛋白S195位点磷酸化,导致PD-L1去糖基化稳定性下降,进而引起其蛋白酶体降解,最终通过降解PD-L1促进免疫治疗和放疗疗效[56-57]。
4.3 B7H3和B7H4糖基化除了PD-L1,同属于B7家族的其他免疫检查点配体分子也以糖基化修饰稳定其细胞表面蛋白表达。B7H3主要表达于肿瘤细胞、抗原提呈细胞和NK细胞表面,有助于肿瘤细胞免疫逃逸[58]。B7H3高表达于口腔鳞癌细胞,其N-聚糖结构呈现高岩藻糖基化,与肿瘤有氧糖酵解及DC特异性细胞间黏附分子-3-抓取非整合素[DC-specific intercellular adhesion molecule-3 (ICAM-3)-grabbing nonintegrin, DC-SIGN]密切相关[59-60]。三阴性乳腺癌中的B7H3也呈现异常的N-糖基化,研究[61]表明其糖基化由α-1, 6-岩藻糖基转移酶(α-1, 6-fucosyltransferase,FUT8)介导,且FUT8的敲减显著降低糖基化B7H3的表达,增强肿瘤免疫应答。B7H4也是一种免疫抑制配体,在肿瘤细胞和肿瘤相关巨噬细胞表面呈高表达状态,但其受体还未明确。Song等[62]发现三阴性乳腺癌免疫“冷肿瘤”中B7H4的表达与PD-L1负相关,B7H4去糖基化降解通过几种“吃我”分子的表达导致免疫原性细胞死亡,改善细胞毒性免疫细胞浸润,完成了“冷肿瘤”向“热肿瘤”的转化。并且在体内外实验中验证了N-糖苷酶联合PD-L1单抗的肿瘤杀伤效果显著优于PD-L1单抗单药治疗。
上述研究成果为后续针对不同免疫检查点糖基化开展应用研究,以及成熟的ICI联合去糖基化酶,提升免疫治疗效果,为肿瘤的临床治疗提供新策略。
5 思考与展望在医学科研创新时代,免疫治疗作为新兴治疗方式改善肿瘤患者的预后。但肿瘤微环境具有异质性[63-64],免疫“冷肿瘤”人群的免疫治疗反应率仍不理想。针对免疫检查点的研究从宏观表达到微观修饰不断深入,揭示了肿瘤微环境内部分免疫调控的机制。诸多证据表明,糖基化修饰介导肿瘤微环境中免疫检查点蛋白的高稳定性及抗体低亲和力表型。但由于糖基化修饰对免疫检查点蛋白的表达及功能调控正反不一,而目前的去糖基化酶(N-糖苷酶F)非特异性地对所有N-糖基化位点进行切割,难以实现精准调控。盲目地采用免疫治疗联合去糖基化的策略在复杂的肿瘤微环境背景下利弊难以权衡。因此,通过去糖基化酶联合高分子化学技术增加特定分子糖基化的策略也许会更有益于提升免疫治疗疗效。除了调控技术的局限性,免疫检查点糖基化的研究范围较窄,主要集中于PD-1及B7家族分子,尚未涉猎其他具有潜在影响肿瘤行为的免疫检查点分子。有必要通过高通量的质谱技术进行人体肿瘤标本免疫相关蛋白糖基化的全面描绘,以便大规模筛选可调控分子并分析去糖基化策略的整体获益情况。
综上所述,靶向免疫检查点糖基化的治疗手段初步展现了显著的疗效及良好应用前景,该干预手段在突破现有桎梏后有望在肿瘤免疫治疗领域做出新的突破并进入临床转化应用。
伦理声明 无。
利益冲突 所有作者声明不存在利益冲突。
作者贡献 周仕钊:思路构想,论文整理,初稿撰写;常文举:初稿的审阅和修改,项目监督和领导,资金支持。
| [1] |
FUSTER M M, ESKO J D. The sweet and sour of cancer: glycans as novel therapeutic targets[J]. Nat Rev Cancer, 2005, 5(7): 526-542.
[DOI]
|
| [2] |
SILSIRIVANIT A. Glycosylation markers in cancer[J]. Adv Clin Chem, 2019, 89: 189-213.
|
| [3] |
HÄUSELMANN I, BORSIG L. Altered tumor-cell glycosylation promotes metastasis[J]. Front Oncol, 2014, 4: 28.
|
| [4] |
RODRIGUES J G, BALMAÑA M, MACEDO J A, et al. Glycosylation in cancer: selected roles in tumour progression, immune modulation and metastasis[J]. Cell Immunol, 2018, 333: 46-57.
[DOI]
|
| [5] |
LI C W, LIM S O, XIA W Y, et al. Glycosylation and stabilization of programmed death ligand-1 suppresses T-cell activity[J]. Nat Commun, 2016, 7: 12632.
[DOI]
|
| [6] |
SUN L L, LI C W, CHUNG E M, et al. Targeting glycosylated PD-1 induces potent antitumor immunity[J]. Cancer Res, 2020, 80(11): 2298-2310.
[DOI]
|
| [7] |
REILY C, STEWART T J, RENFROW M B, et al. Glycosylation in health and disease[J]. Nat Rev Nephrol, 2019, 15(6): 346-366.
[DOI]
|
| [8] |
HU P, SHIMOJI S, HART G W, et al. Site-specific interplay between O-GlcNAcylation and phosphorylation in cellular regulation[J]. FEBS Lett, 2010, 584(12): 2526-2538.
[DOI]
|
| [9] |
MUSICKI B, KRAMER M F, BECKER R E, et al. Inactivation of phosphorylated endothelial nitric oxide synthase (Ser-1177) by O-GlcNAc in diabetes-associated erectile dysfunction[J]. Proc Natl Acad Sci USA, 2005, 102(33): 11870-11875.
[DOI]
|
| [10] |
OGATA S I, MURAMATSU T, KOBATA A. New structural characteristic of the large glycopeptides from transformed cells[J]. Nature, 1976, 259(5544): 580-582.
[DOI]
|
| [11] |
HAKOMORI S, KANNAGI R. Glycosphingolipids as tumor-associated and differentiation markers[J]. J Natl Cancer Inst, 1983, 71(2): 231-251.
|
| [12] |
KUMAMOTO K, GOTO Y, SEKIKAWA K, et al. Increased expression of UDP-galactose transporter messenger RNA in human colon cancer tissues and its implication in synthesis of Thomsen-Friedenreich antigen and sialyl Lewis A/X determinants[J]. Cancer Res, 2001, 61(11): 4620-4627.
|
| [13] |
BUCKHAULTS P, CHEN L, FREGIEN N, et al. Transcriptional regulation of N-acetylglucosaminyltransferase Ⅴ by the src oncogene[J]. J Biol Chem, 1997, 272(31): 19575-19581.
[DOI]
|
| [14] |
ARYAL R P, JU T Z, CUMMINGS R D, et al. Identification of a novel protein binding motif within the T-synthase for the molecular chaperone Cosmc[J]. J Biol Chem, 2014, 289(17): 11630-11641.
[DOI]
|
| [15] |
KELLOKUMPU S, SORMUNEN R, KELLOKUMPU I. Abnormal glycosylation and altered Golgi structure in colorectal cancer: dependence on intra-Golgi pH[J]. FEBS Lett, 2002, 516(1/2/3): 217-224.
|
| [16] |
SHI J, TOMAŠIČ T, SHARIF S, et al. Peptide microarray analysis of the cross-talk between O-GlcNAcylation and tyrosine phosphorylation[J]. FEBS Lett, 2017, 591(13): 1872-1883.
[DOI]
|
| [17] |
LÄUBLI H, BORSIG L. Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis[J]. Front Immunol, 2019, 10: 2120.
[DOI]
|
| [18] |
RUAN Y S, CHEN L B, XIE D F, et al. Mechanisms of cell adhesion molecules in endocrine-related cancers: a concise outlook[J]. Front Endocrinol (Lausanne), 2022, 13: 865436.
[DOI]
|
| [19] |
CARVALHO S, CATARINO T A, DIAS A M, et al. Preventing E-cadherin aberrant N-glycosylation at Asn-554 improves its critical function in gastric cancer[J]. Oncogene, 2016, 35(13): 1619-1631.
[DOI]
|
| [20] |
PINHO S S, REIS C A, PAREDES J, et al. The role of N-acetylglucosaminyltransferase Ⅲ and Ⅴ in the post-transcriptional modifications of E-cadherin[J]. Hum Mol Genet, 2009, 18(14): 2599-2608.
[DOI]
|
| [21] |
SARRAZIN S, LAMANNA W C, ESKO J D. Heparan sulfate proteoglycans[J]. Cold Spring Harb Perspect Biol, 2011, 3(7): a004952.
|
| [22] |
WADE A, ROBINSON A E, ENGLER J R, et al. Proteoglycans and their roles in brain cancer[J]. Febs J, 2013, 280(10): 2399-2417.
[DOI]
|
| [23] |
CECCHI F, PAJALUNGA D, FOWLER C A, et al. Targeted disruption of heparan sulfate interaction with hepatocyte and vascular endothelial growth factors blocks normal and oncogenic signaling[J]. Cancer Cell, 2012, 22(2): 250-262.
[DOI]
|
| [24] |
LIBERTI M V. The Warburg effect: how does it benefit cancer cells?[J]. Trends Biochem Sci, 2016, 41(3): 211-218.
[DOI]
|
| [25] |
WELLS L, VOSSELLER K, HART G W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc[J]. Science, 2001, 291(5512): 2376-2378.
[DOI]
|
| [26] |
BUTKINAREE C, et al. O-linked β-N-acetylglucosamine (O-GlcNAc): extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress[J]. Biochim Biophys Acta, 2010, 1800(2): 96-106.
[DOI]
|
| [27] |
ITKONEN H M, URBANUCCI A, MARTIN S E, et al. High OGT activity is essential for MYC-driven proliferation of prostate cancer cells[J]. Theranostics, 2019, 9(8): 2183-2197.
[DOI]
|
| [28] |
YANG W H, KIM J E, NAM H W, et al. Modification of p53 with O-linked N-acetylglucosamine regulates p53 activity and stability[J]. Nat Cell Biol, 2006, 8(10): 1074-1083.
[DOI]
|
| [29] |
KIM S H, TURNBULL J, GUIMOND S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor[J]. J Endocrinol, 2011, 209(2): 139-151.
[DOI]
|
| [30] |
RABINOVICH G A, TOSCANO M A. Turning 'sweet' on immunity: galectin–glycan interactions in immune tolerance and inflammation[J]. Nat Rev Immunol, 2009, 9(5): 338-352.
[DOI]
|
| [31] |
FRASCHILLA I, PILLAI S. Viewing Siglecs through the lens of tumor immunology[J]. Immunol Rev, 2017, 276(1): 178-191.
[DOI]
|
| [32] |
ANGATA T. Possible influences of endogenous and exogenous ligands on the evolution of human siglecs[J]. Front Immunol, 2018, 9: 2885.
[DOI]
|
| [33] |
PILLAI S, NETRAVALI I A, CARIAPPA A, et al. Siglecs and immune regulation[J]. Annu Rev Immunol, 2012, 30: 357-392.
[DOI]
|
| [34] |
KARMAKAR J, MUKHERJEE K, MANDAL C. Siglecs modulate activities of immune cells through positive and negative regulation of ROS generation[J]. Front Immunol, 2021, 12: 758588.
[DOI]
|
| [35] |
TANIDA S, AKITA K, ISHIDA A, et al. Binding of the sialic acid-binding lectin, Siglec-9, to the membrane mucin, MUC1, induces recruitment of β-catenin and subsequent cell growth[J]. J Biol Chem, 2013, 288(44): 31842-31852.
[DOI]
|
| [36] |
LÄUBLI H, PEARCE O M, SCHWARZ F, et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer[J]. Proc Natl Acad Sci USA, 2014, 111(39): 14211-14216.
[DOI]
|
| [37] |
HUDAK J E, CANHAM S M, BERTOZZI C R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion[J]. Nat Chem Biol, 2014, 10(1): 69-75.
[DOI]
|
| [38] |
MERIL S, HARUSH O, REBOH Y, et al. Targeting glycosylated antigens on cancer cells using siglec-7/9-based CAR T-cells[J]. Mol Carcinog, 2020, 59(7): 713-723.
[DOI]
|
| [39] |
VAN HOUTUM E J H, BÜLL C, CORNELISSEN L A M, et al. Siglec signaling in the tumor microenvironment[J]. Front Immunol, 2021, 12: 790317.
[DOI]
|
| [40] |
SANTEGOETS K C M, GIELEN P R, BÜLL C, et al. Expression profiling of immune inhibitory Siglecs and their ligands in patients with glioma[J]. Cancer Immunol Immunother, 2019, 68(6): 937-949.
[DOI]
|
| [41] |
HAAS Q, BOLIGAN K F, JANDUS C, et al. Siglec-9 regulates an effector memory CD8+ T-cell subset that congregates in the melanoma tumor microenvironment[J]. Cancer Immunol Res, 2019, 7(5): 707-718.
[DOI]
|
| [42] |
KALBASI A, RIBAS A. Tumour-intrinsic resistance to immune checkpoint blockade[J]. Nat Rev Immunol, 2020, 20(1): 25-39.
[DOI]
|
| [43] |
LITTMAN D R. Releasing the brakes on cancer immunotherapy[J]. Cell, 2015, 162(6): 1186-1190.
[DOI]
|
| [44] |
BARECHE Y, BUISSERET L, GRUOSSO T, et al. Unraveling triple-negative breast cancer tumor microenvironment heterogeneity: towards an optimized treatment approach[J]. J Natl Cancer Inst, 2020, 112(7): 708-719.
[DOI]
|
| [45] |
POSTOW M A, SIDLOW R, HELLMANN M D. Immune-related adverse events associated with immune checkpoint blockade[J]. N Engl J Med, 2018, 378(2): 158-168.
[DOI]
|
| [46] |
ZHANG S, WANG L, LI M T, et al. The PD-1/PD-L pathway in rheumatic diseases[J]. J Formos Med Assoc, 2021, 120(1 Pt 1): 48-59.
|
| [47] |
FIFE B T, PAUKEN K E. The role of the PD-1 pathway in autoimmunity and peripheral tolerance[J]. Ann N Y Acad Sci, 2011, 1217: 45-59.
[DOI]
|
| [48] |
OKADA M, CHIKUMA S, KONDO T, et al. Blockage of core fucosylation reduces cell-surface expression of PD-1 and promotes anti-tumor immune responses of T cells[J]. Cell Rep, 2017, 20(5): 1017-1028.
[DOI]
|
| [49] |
WANG M, WANG J, WANG R, et al. Identification of a monoclonal antibody that targets PD-1 in a manner requiring PD-1 Asn58 glycosylation[J]. Commun Biol, 2019, 2: 392.
[DOI]
|
| [50] |
LU D, XU Z P, ZHANG D, et al. PD-1 N58-glycosylation-dependent binding of monoclonal antibody cemiplimab for immune checkpoint therapy[J]. Front Immunol, 2022, 13: 826045.
[DOI]
|
| [51] |
LIU K F, TAN S G, JIN W J, et al. N-glycosylation of PD-1 promotes binding of camrelizumab[J]. EMBO Rep, 2020, 21(12): e51444.
[DOI]
|
| [52] |
GRECO B, MALACARNE V, DE GIRARDI F, et al. Disrupting N-glycan expression on tumor cells boosts chimeric antigen receptor T cell efficacy against solid malignancies[J]. Sci Transl Med, 2022, 14(628): eabg3072.
[DOI]
|
| [53] |
XU J Y, YANG X J, MAO Y, et al. Removal of N-linked glycosylation enhances PD-L1 detection in colon cancer: validation research based on immunohistochemistry analysis[J]. Technol Cancer Res Treat, 2021, 20: 15330338211019442.
|
| [54] |
LEE H H, et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts anti-PD-1/PD-L1 therapeutic efficacy[J]. Cancer Cell, 2019, 36(2): 168-178.e4.
|
| [55] |
LI C W, LIM S O, CHUNG E M, et al. Eradication of triple-negative breast cancer cells by targeting glycosylated PD-L1[J]. Cancer Cell, 2018, 33(2): 187-201.e10.
|
| [56] |
ZHANG R N, YANG Y J, DONG W J, et al. D-mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1[J]. Proc Natl Acad Sci USA, 2022, 119(8): e2114851119.
|
| [57] |
CHA J H, YANG W H, XIA W Y, et al. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1[J]. Mol Cell, 2018, 71(4): 606-620.e7.
|
| [58] |
FLEM-KARLSEN K, FODSTAD Ø, NUNES-XAVIER C E. B7-H3 immune checkpoint protein in human cancer[J]. Curr Med Chem, 2020, 27(24): 4062-4086.
|
| [59] |
LI Z G, LIU J Y, QUE L, et al. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway[J]. J Cancer, 2019, 10(23): 5770-5784.
|
| [60] |
CHEN J T, CHEN C H, KU K L, et al. Glycoprotein B7-H3 overexpression and aberrant glycosylation in oral cancer and immune response[J]. Proc Natl Acad Sci USA, 2015, 112(42): 13057-13062.
|
| [61] |
CHOI I H, ZHU G F, SICA G L, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family[J]. J Immunol, 2003, 171(9): 4650-4654.
|
| [62] |
SONG X X, ZHOU Z, LI H C, et al. Pharmacologic suppression of B7-H4 glycosylation restores antitumor immunity in immune-cold breast cancers[J]. Cancer Discov, 2020, 10(12): 1872-1893.
|
| [63] |
储钜航, 钱明平. 外泌体在恶性肿瘤发生发展中的作用及临床应用[J]. 同济大学学报(医学版), 2022, 43(5): 734-740. CHU J H, QIAN M P. Exosomes in the occurrence and development of cancer and its clinical application[J]. J Tongji Uni (Med Sci), 2022, 43(5): 734-740. [CNKI] |
| [64] |
许博, 孙梦瑶, 吴秋雪, 等. 外泌体蛋白质在消化系统恶性肿瘤中的研究进展[J]. 同济大学学报(医学版), 2021, 42(1): 109-115. XU B, SUN M Y, WU Q X, et al. Progress of exosomal protein in malignant tumors of digestive system[J]. J Tongji Uni (Med Sci), 2021, 42(1): 109-115. [CNKI] |
 2024, Vol. 31
2024, Vol. 31


