2. 复旦大学附属中山医院放射科, 上海市影像医学研究所, 上海 200032;
3. 复旦大学附属中山医院病理科, 上海 200032
2. Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai institute of imaging Medicine, Shanghai 200032, China;
3. Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai 200032, China
胰腺神经内分泌肿瘤(pancreatic neuroendo-crine neoplasms,pNEN)是一类生物学行为呈高度异质性的肿瘤,既可惰性生长,亦可侵袭性生长,甚至早期发生转移,其生物学行为可能随着疾病的进展而变化。近年来,pNEN的发病率明显上升,随着检查技术的进步和健康体检的普及,pNEN的临床检出率亦呈上升趋势[1]。WHO 2017年消化系统肿瘤分类标准[2]将pNEN分为分化好的胰腺神经内分泌肿瘤(pancreatic neuroendocrine tumors,pNET)和分化差的胰腺神经内分泌癌(pancreatic neuroendocrine carcinoma,pNEC)。不同病理级别pNEN的生物学行为各异,患者的预后差别显著。因此,准确判断pNEN的病理分级,有助于临床治疗方案的制订和患者的预后评估。
本课题组前期研究[3-4]发现,肿瘤的大小、形状、边缘、MRI信号均质程度、胰腺外侵犯和转移等有助于术前预测pNEN的病理分级。弥散加权成像(diffusion weighted imaging, DWI)可以通过人体组织中水分子布朗运动的受限差异,来反映不同组织的信息。其量化参数表观弥散系数(apparent diffusion coefficient, ADC)可定量反映组织内细胞的密集程度,可应用于良恶性病变的鉴别诊断、预后判断及疗效评估等。本研究探讨ADC对pNEN术前病理分级的预测价值。
1 资料与方法 1.1 一般资料回顾性分析2015年3月至2019年6月复旦大学附属中山医院诊治的pNEN患者72例,其中男性43例、女性29例,年龄15~77岁,平均年龄(54±15)岁。30例患者有临床症状,包括低血糖综合征5例、腹痛或腹胀不适23例、黄疸2例;42例患者无明显临床症状。
纳入标准:(1)经手术病理确诊为pNEN;(2)术前进行MRI常规和DWI扫描;(3)MRI检查前未接受过其他治疗;(4)MRI检查与手术间隔时间不超过1个月。排除标准:(1)图像质量不佳,不能用于诊断和分析;(2)肿瘤太小,导致ADC的测量受容积效应影响。
1.2 MRI检查方法MR检查仪包括Siemens Magnetom Avanto 1.5 Tesla超导MR机、Siemens Magnetom Aera 1.5 Tesla超导MR机。所有患者均进行常规MRI平扫、增强扫描和DWI扫描。平扫序列包括脂肪抑制屏气快速自旋回波T2WI、梯度回波正反相位T1WI;增强扫描采用三维容积间插重建梯度回波抑脂T1WI序列,对比剂采用Gd-DTPA(剂量为0.1 mmol/kg);DWI扫描采用呼吸门控技术,横断面单次激发平面回波成像序列:TR 8 000 ms,TE 67 ms,层厚5 mm,层间距1 mm,FOV 380×380 mm,b值选取0和800 s/mm2。
1.3 图像分析由2名具有10年以上腹部MRI诊断经验的放射科医师在仅知晓患者为pNEN、不知晓手术及病理分级结果的情况下,通过PACS系统共同阅片,观察瘤灶特征,并达成一致意见。在后处理工作站上首先结合平扫、DWI及增强图像明确胰腺中瘤灶的位置,然后在DWI图像上对应的瘤灶实性区域测量瘤灶ADC。ADC测量方法:选择信号最低的肿瘤区,尽量避开囊变、坏死区域,手动绘制感兴趣区域(ROI),测量2次ADC,取平均值;在瘤灶上下层面且远离发病部位的胰腺实性区域勾画ROI,测量2次胰腺ADC,取平均值。
1.4 病理分析参照WHO 2017年消化系统肿瘤分类标准[2],将pNEN按照肿瘤细胞增殖指数Ki-67和有丝分裂指数分为pNET G1组、pNET G2组、pNET G3组和pNEC G3组(表 1)。记录患者术后免疫组化病理报告中瘤灶的Ki-67值。
| 分类 | 分组 | Ki-67/% | 有丝分裂指数(个/10HPF) |
| pNET | pNET G1组 | <3 | <2 |
| pNET G2组 | 3~20 | 2~20 | |
| pNET G3组 | >20 | >20 | |
| pNEC | pNEC G3组 | >20 | >20 |
采用SPSS 24.0软件进行统计分析。经Kolmogorov-Smirnov检验,所有纳入计量资料符合正态分布,以x±s表示,采用单因素方差分析。采用Spearman法分析pNEN瘤灶ADC与Ki-67的相关性;采用LSD法进行各组间瘤灶ADC、胰腺ADC和Ki-67的两两比较;采用ROC分析pNEN瘤灶ADC对病理分级的诊断效能。检验水准(α)为0.05。
2 结果 2.1 pNEN的ADC和Ki-67值的比较72例pNEN均为单发,pNET G1组18例、pNET G2组36例、pNET G3组13例、pNEC G3组5例。结果(表 2)显示:4组间瘤灶ADC差异有统计学意义(F=75.474,P < 0.001),胰腺ADC差异无统计学意义(F=1.257,P=0.296),瘤灶Ki-67值差异有统计学意义(F=108.653,P < 0.001)。图 1示不同病理级别组pNEN的影像学表现。
| 组别 | 瘤灶ADC×10-3/(mm2·s-1) | 胰腺ADC×10-3/(mm2·s-1) | 瘤灶Ki-67/% |
| pNET G1组(n=18) | 1.79±0.19 | 2.04±0.23 | 1.47±0.49 |
| pNET G2组(n=36) | 1.39±0.14 | 1.98±0.16 | 7.22±4.73 |
| pNET G3组(n=13) | 1.04±0.15 | 1.93±0.12 | 38.46±15.46 |
| pNEC G3组(n=5) | 0.94±0.11 | 1.96±0.14 | 62.00±17.89 |
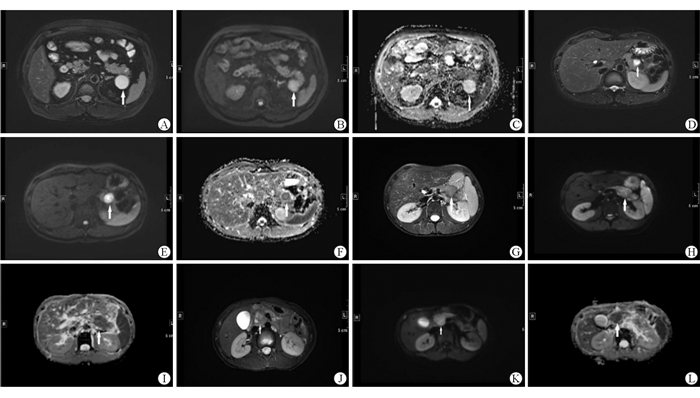
|
| 图 1 各病理级别pNEN的T2WI、DWI和ADC A~C: pNET G1,瘤灶ADC 1.882×10-3 mm2/s,Ki-67为2%; D~F: pNET G2,瘤灶ADC 1.335×10-3 mm2/s,Ki-67为5%; G~I: pNET G3,瘤灶ADC 0.962 ×10-3 mm2/s,Ki-67为25%; J~L: pNEC G3,瘤灶ADC 0.937×10-3 mm2/s,Ki-67为60%。 |
pNET G1组与pNET G2组、pNET G1组与pNET G3组、pNET G1组与pNEC G3组、pNET G2组与pNET G3组、pNET G2组与pNEC G3组间瘤灶ADC差异均有统计学意义(P < 0.001);pNET G3组与pNEC G3组间瘤灶ADC差异无统计学意义(P=0.233)。
2.2 pNEN瘤灶ADC与Ki-67的相关性结果(图 2)显示:pNEN瘤灶ADC与Ki-67负相关(r=-0.845,P < 0.001)。
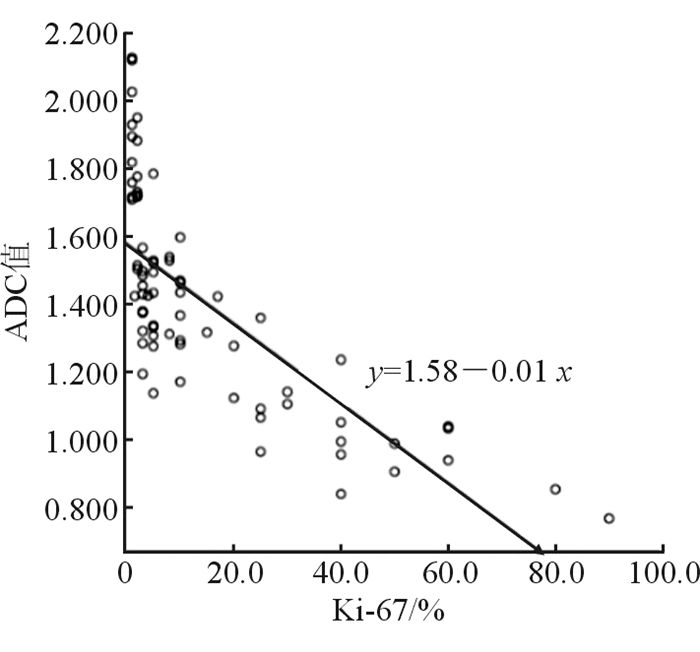
|
| 图 2 pNEN瘤灶ADC与Ki-67相关性 |
结果(图 3)显示:ADC以1.596×10-3 mm2/s为界值,区分pNET G1与G2的灵敏度为97.22%、特异度为83.33%,AUC为0.941(Z=13.340,P < 0.001);ADC以1.103×10-3 mm2/s为界值,区分pNET G2与G3的灵敏度为83.33%、特异度为100%,AUC为0.968(Z=18.830,P < 0.001)。在G3级pNEN中,分化好的pNET G3与分化差的pNEC G3组间瘤灶ADC差异无统计学意义,故无法以瘤灶ADC来区分pNET G3与pNEC G3。
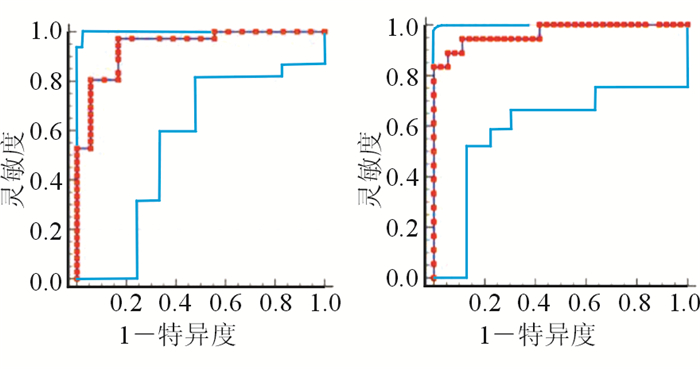
|
| 图 3 术前瘤灶ADC对pNEN分级的ROC曲线 A: ADC区分pNEN G1与G2; B: ADC区分pNEN G2与G3。 |
所有胰腺神经内分泌肿瘤均具有不同程度的恶性潜能,随着病理级别的增高,其恶性程度升高。pNEN的病理级别与其生物学行为、患者的预后及临床治疗方案的选择密切相关[5-7]。
本研究结果表明,随着pNEN病理级别的增高,瘤灶ADC呈现递降趋势,与肿瘤细胞增殖指数Ki-67负相关,与国内外相关研究[8-10]结果一致。pNEN瘤灶与Ki-67负相关的原因如下:(1)高级别pNEN细胞的增殖速度更快,瘤体内细胞数目增多、细胞排列更加紧密,使细胞周围间隙减小,导致水分子自由弥散受到限制;(2)不同病理级别pNEN肿瘤细胞的分化差异使细胞内核浆比例改变,导致水分子弥散运动的受限程度不同。
本研究结果显示,高级别与低级别pNEN瘤灶ADC的差异存在统计学意义,ADC以1.596×10-3 mm2/s为界值区分pNET G1与G2的灵敏度和特异度高,具有较高的诊断效能;ADC以1.103×10-3 mm2/s为界值区分pNET G2与G3的灵敏度和特异度较高,也具有较高的诊断效能。因此,采用ADC有助于术前pNEN病理分级的评估。此研究结果与国内外相关文献[8, 11-12]报道相符。对于无法手术治疗的pNEN患者,准确预测肿瘤的病理分级有助于制订精准治疗方案,改善患者预后。此外,本研究中,pNET G3与pNEC G3瘤灶ADC差异无统计学意义,可能与pNEC G3发病率较低,导致纳入样本量偏少有关,有待于今后加大样本量进一步研究。
本研究不足之处:(1)由于是回顾性研究,扫描数据并不来源于同一台MR机,对ADC测值可能产生一定影响;(2)部分病例肿瘤体积较小或内部囊变、坏死成分较多,ADC测量受到影响。
综上所述,不同病理级别pNEN的ADC差异有统计学意义,与Ki-67负相关,ADC可区分低级别和高级别的pNEN,因此术前可采用ADC预测pNEN的病理分级。但是由于ADC测量易受到多种因素影响,今后可选择肿瘤信号与胰腺正常实质信号的比值等更客观的指标,以提高术前评估pNEN病理分级的准确性。
利益冲突:所有作者声明不存在利益冲突。
| [1] |
吴文铭, 陈洁, 白春梅, 等. 中国胰腺神经内分泌诊疗指南(2020)[J]. 中华外科杂志, 2021, 59(6): 401-421. WU W M, CHEN J, BAI C M, et al. Guidelines for diagnosis and treatment of pancreatic neuroendocrine in China (2020)[J]. Chinese Journal of Surgery, 2021, 59(6): 401-421. [DOI] |
| [2] |
SUNDIN A, ARNOLD R, BAUDIN E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging[J]. Neuroendocrinology, 2017, 105(3): 212-244.
[DOI]
|
| [3] |
WANG M L, JI Y S, XIE Y H, et al. MRI appearance of pancreatic neuroendocrine neoplasms correlation with pathological grade: comparative study[J]. Chin J Radiol, 2017(2): 136-140.
|
| [4] |
姚秀忠, 刘豪, 陈文芳, 等. 3.0T磁共振自由呼吸背景抑制弥散加权成像对胰腺肿块的诊断价值[J]. 中国临床医学, 2013, 20(1): 64-66. YAO X Z, LIU H, CHEN W F, et al. Value of free-breathing diffusion-weighted mr imaging with background suppression by inversion recovery at 3.0T for the diagnosis of solid pancreatic masses[J]. Chinese Journal of Clinical Medicine, 2013, 20(1): 64-66. [CNKI] |
| [5] |
GUILMETTE J M, NOSÉ V. Neoplasms of the neuroendocrine pancreas: an update in the classification, definition, and molecular genetic advances[J]. Adv Anat Pathol, 2019, 26(1): 13-30.
[DOI]
|
| [6] |
PARTELLI S, BARTSCH D K, CAPDEVILA J, et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: surgery for small intestinal and pancreatic neuroendocrine tumours[J]. Neuroendocrinology, 2017, 105(3): 255-265.
[DOI]
|
| [7] |
BASTURK O, YANG Z H, TANG L H, et al. The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms[J]. Am J Surg Pathol, 2015, 39(5): 683-690.
[DOI]
|
| [8] |
孙素, 杨爱春, 付玉川, 等. 胰腺神经内分泌肿瘤的MRI表现与病理分级的对照研究[J]. 中华内分泌外科杂志, 2017, 11(6): 490-493, 499. SUN S, YANG A C, FU Y C, et al. A comparative study of MRI findings and pathological grading of pancreatic neuroendocrine tumors[J]. Chinese Journal of Endocrine Surgery, 2017, 11(6): 490-493, 499. [DOI] |
| [9] |
TOSHIMA F, INOUE D, KOMORI T, et al. Is the combination of MR and CT findings useful in determining the tumor grade of pancreatic neuroendocrine tumors[J]. Jpn J Radiol, 2017, 35(5): 242-253.
[DOI]
|
| [10] |
JANG K M, KIM S H, LEE S J, et al. The value of gadoxetic acid-enhanced and diffusion-weighted MRI for prediction of grading of pancreatic neuroendocrine tumors[J]. Acta Radiol, 2014, 55(2): 140-148.
[DOI]
|
| [11] |
LOTFALIZADEH E, RONOT M, WAGNER M, et al. Prediction of pancreatic neuroendocrine tumour grade with MR imaging features: added value of diffusion-weighted imaging[J]. Eur Radiol, 2017, 27(4): 1748-1759.
[DOI]
|
| [12] |
ZONG R L, GENG L, WANG X, et al. Diagnostic performance of apparent diffusion coefficient for prediction of grading of pancreatic neuroendocrine tumors: a systematic review and meta-analysis[J]. Pancreas, 2019, 48(2): 151-160.
[DOI]
|
 2021, Vol. 28
2021, Vol. 28


